 Care Delivery
Care Delivery
Next Generation Digital Care & Data Technologies
Innovating Musculoskeletal Care
Explore some of our patented technologies within our three verticals. These are available for licensing and collaborations as we are continuously seeking commercial partnerships.
Next Generation Digital Care & Data Technologies
Device & Instrumentation Development
Drugs, Biologics, & Research Innovations
IV catheter delivery system that includes a mechanism to prevent back-walling while continuously monitoring the flash of blood
Inventor:
Michael Singleton, MD
Status: Patent Pending
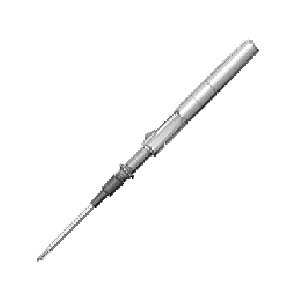
Clinical Problem: There have been minimal design features to help novices achieve high rates of first attempt success when inserting and starting an IV. The practitioner must advance the needle further forward (a distance which depends on how big the needle is and how far the catheter tip is from the needle point) until the catheter is in the vein. This additional advancement runs the risk of “back-walling” the vein, or the needle point puncturing the other side of the vein and thus disrupting the integrity of the vein. Therefore, there is a need for an IV catheter device that addresses and overcomes the above noted deficiencies and one that allows for proper placement of the catheter without the risk of “back-walling” the vein.
Solution: This invention allows a practitioner to safely advance the needle forward until the catheter tip is also within vessel lumen without fearing penetration of the needle through the posterior wall of the vessel. This invention would likely be most beneficial for vessels which require steep angles of attack, such as ultrasound guided peripheral IVs placed in the obese patient.
Application: This invention can be a stand-alone product or integrated into an existing peripheral or arterial catheter system or other vascular access procedures.
System and method for localized delivery of medications/fluids to a wound site over extended periods of time with minimal effect on the patient’s mobility
Inventor:
Stavros Memtsoudis, MD, PhD
Status: Patents Granted, Patents Pending
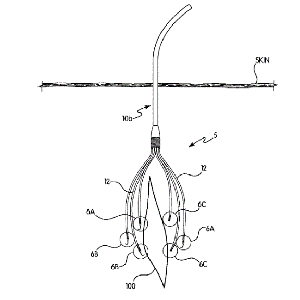
Clinical Problem: Post-operative pain management for joint replacement patients has improved significantly in the past 10-15 years with the use of various anesthetic/analgesic “cocktails” injected into the surgical site at the end of the procedure. However, despite their efficacy in reducing immediate post-op pain and facilitating mobility, the effects have a limited time of action. Further pain management might require multiple intra-articular injections (for which the length of action, duration, or strength cannot be controlled) or use of powerful oral drugs (which may introduce further complications of dependency).
Solution: A system that enables controlled, targeted delivery of medications to a wound site over time. A main flexible cannula that has multiple smaller cannulas coming off it, each of which has perforations that enable flow of medication/fluid to the surrounding area. Each small cannula terminates with a hook that is used to affix in a specific area of tissue.
Application: The Multi-Catheter Infusion System has multiple catheters that can be placed in a wider, more targeted region of treatment (e.g., multiple areas of the intra-articular space within the knee).
This invention can be integrated as part of an infusion pump system, as a holistic protocol for post-operative pain management.
A delivery vehicle designed to be a transport platelet rich plasma (PRP) and/or mineral rich compounds
Inventor:
William Rodriguez
Status: Patent Granted
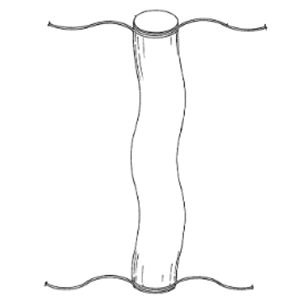
Clinical Problem: Surgical sites, whether arthroscopic, endoscopic or open surgery, are closed via sutures or staples. It is also known that certain biological products aid in the healing and regenerative process of such surgical sites. The conventional practice is to coat or cover standard sutures with biological products to deliver these biologics to the surgical site. However, such a method of delivering biologics to a surgical site does not result in an effective amount of biological material being delivered, nor does it provide a means to deliver additional biologics after suturing, or the ability to adequately deliver high viscous biological compounds.
Solution: A delivery vehicle, made of flexible porous film container, designed to be a transport for platelet rich plasma (PRP) and/or mineral rich compounds that have been enhanced for the use in regenerative procedures and/or antibiotics to specific sites determined by the surgical team. The container is moveable between an open position and a closed position, a first compartment in fluid communication with the access, and a second compartment in fluid communication with the access; a filter covering the access; and a fastener for securing the sterile, flexible, porous film container adjacent to the surgical site, wherein the container is moveable between open and closed positions via the fastener.
Application: This system is a stand-alone product line that could accompany a biologic or suture system.
Spring loaded cleat assembly to enhance rotational and translational release to minimize the occurrence of soft tissue injuries
Inventor:
Hernan Sanchez
Laurence Piturro
Bo Li
Mark Drakos, MD
Howard Hillstrom, PhD
Andrew Kraszewski, PhD
Status: Patent Granted
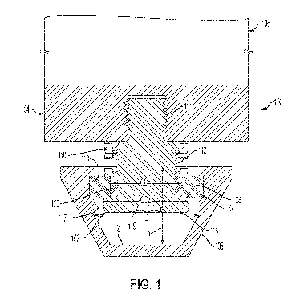
Clinical Problem: Shoe cleat assemblies that permit axial movement of the cleat with respect to the shoe are known. Such assemblies enable the cleat to move along a longitudinal axis of the cleat. However, such assemblies are limited to only movement along a single degree of freedom.
Solution: A cleat assembly having multiple biasing members to permit movement of the cleat about multiple degrees of freedom. The cleat assembly provides effective axial shock absorbance coupled with 360° tilting of the cleat for enhancing a user's ability to suddenly change direction when wearing a shoe equipped with the cleat assembly, thereby minimizing stress and impact on muscles, joints and ligaments and enhancing the performance of athletes wearing such shoes.
Application: This invention can be a stand-alone product or integrated into existing athletic shoes.
Device to assess first ray position and mobility, arch height flexibility and stiffness, and first metatarsophalangeal joint flexibility
Inventor:
Jonathan Deland, MD
Scott J. Ellis, MD
Howard Hillstrom, PhD
Rajshree Hillstrom, PhD
Oliver Morgan, PhD
Jinsup Song
Robert Turner
Status: Patents Pending
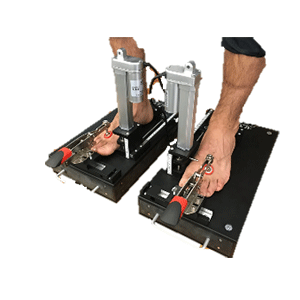
Clinical Problem: The “first ray” refers to a segment of a patient’s foot composed of a navicular, medial cuneiform, first metatarsal, and hallux bones. Aberrant first ray structure and function have been implicated in onset and progression of osteoarthritis in the first metatarsophalangeal joint, sometimes referred to as hallux rigidus. Specifically, investigation is ongoing regarding the impact of hypermobility of the first ray in the superior direction, measured by first ray mobility, on hallux rigidus.
Conventional assessment devices are operable to quantify first ray mobility objectively. However, such devices tend to yield inaccurate measurements, in part due to requiring manual force during at least part of the examination, thereby potentially adding undesired variability to the measurement. Conventional devices can also be complicated and bulky, thereby limiting their utility in a clinical or research setting.
Solution: Device to assess first ray position, first ray mobility, arch height index, arch height flexibility, arch stiffness, and first metatarsophalangeal joint flexibility.
Application: This system is a stand-alone product that can be integrated into any clinical or research setting.
System of implants and instruments designed for treatment of arthritis of the subtalar joint
Inventor:
Constantine Demetracopolous, MD
Daniel R. Sturnick, MS
Status: Patents Pending
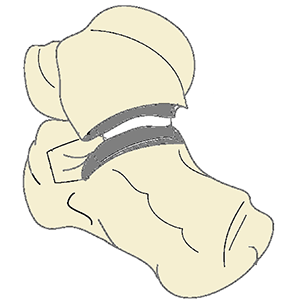
Clinical Problem: Subtalar joint arthritis commonly accompanies arthritis of the ankle, although isolated subtalar arthritis can result in post-traumatic patients. The effect of subtalar arthritis is typically pain and abnormal gait. The most common treatment is fusion of the joint via bone screws. However non-union rates of up to 45% have been reported. It has also been reported that subtalar fusion has a direct relationship to and can be a predictor for total ankle arthroplasty (TAA) failure, in cases where subtalar fusion was done in conjunction with TAA. Providing an option where the effects of subtalar arthritis can be treated while maintaining mobility of the subtalar joint is needed.
Solution: As an alternative to fusion and potentially improving ankle kinematics and overall gait, the implant system is designed to replace the subtalar articular portions of the talus and calcaneous. The associated instrumentation leverages patient-specific guide techniques and minimally invasive instruments for bone preparation and placement of implants.
Application: This system is a stand-alone product line that can be integrated into an ankle arthritis treatment portfolio.
Improved method(s) and instrument(s) for orienting the acetabular cup as part of a total hip replacement
Inventor:
David Mayman, MD
Morteza Meftah, MD
Chitranjan D. Ranawat, MD
Joseph Lipman, MS
Status: Patents Granted
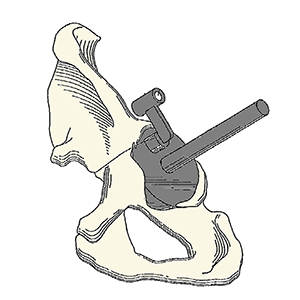
Clinical Problem: In surgery, correct positioning of implants with respect to the patient's anatomy is often a critical factor in achieving a successful outcome. During a total hip replacement (THR), proper implantation of the acetabular cup is challenging. The optimal orientation of the surgical implant enhances the initial function and the long-term operability of the implant. From a biomechanical standpoint, successful hip function depends on a number of factors including the alignment of an acetabular cup. Accurate orienting of the cup is an important variable in reducing the risk of dislocation, bearing impingement, wear and edge loading, revisions, and long-term survivorship of the THR.
Solution: The Acetabular Positioning System includes an improved method(s) and instrument(s) for orienting the acetabular component (cup) during and as part of a total hip replacement procedure. This system takes into account the patient's pelvic position on the operating table, degenerative lumbosacral disease, pelvic tilt, and the presence of osteophytes.
Application: This system can be integrated into an existing hip arthroplasty portfolio.
Set of instruments and guides for use in femoroacetabular surgical/arthroscopic procedures
Inventor:
Friedrich Boettner, MD
Joseph Lipman, MS
Status: Patents Granted
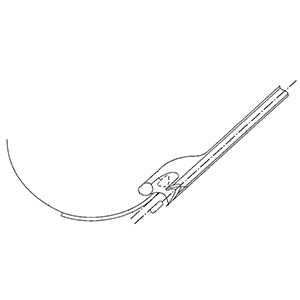
Clinical Problem: Combined femoral (Cam) and acetabular (Pincer) impingement are found in the majority of hips with femoroacetabular impingement.
Femoroacetabular impingement is associated with cartilage damage, labral tears, early hip arthritis, and low back pain, and while femoroacetabular impingement is common in high level athletes, it also occurs in active individuals as well as others.
With the recognition of femoroacetabular impingement as a source of cartilage damage and arthritis, new treatment options have been proposed and developed over the last decade. Despite the recent improvements in treating femoroacetabular impingement, there is a need to provide improved instruments and techniques that can be used to treat femoroacetabular impingement of both types.
Solution: Improved instruments (tools) and surgical techniques for use in surgical procedures that treat femoroacetabular impingement of both the Cam and Pincer types.
Application: This system is a stand-alone product that can be integrated into any surgical instrumentation kit.
Inventor:
Donald Bartel, PhD
Timothy Wright, PhD
Joseph Lipman, MS
Status: Patents Granted
Clinical Problem: Total Knee Arthroplasty (TKA) devices can fail for reasons such as aseptic loosening, instability, or infection. Failure usually requires revision surgery. Revision implants have been developed that include a post on the polyethylene tibial component that articulates within a recess (intercondylar box) in the femoral component. The objective of this so called constrained condylar knee (CCK) implant is to rely on contact between the box and the post within the joint itself to restrain and limit rotation of the knee (varus/valgus rotations). This constraint is also beneficial in primary TKA if the soft tissues cannot be balanced to achieve an adequately stabilized and controlled joint.
Solution: A constrained condylar knee implant system for primary and revision surgery that includes nontoroidal surface geometry on the joint bearing surfaces to provide improved stability and performance of the joint during rotation (varus/valgus). Additional features of the device tend to reduce contact stress on the post improving longevity and functionality of the joint.
Application: This system is a stand-alone product that can be integrated into a TKA portfolio.
System and method for gap assessment examination and surgical assistance in total knee arthroplasty
Inventor:
Cynthia Kahlenberg, MD
Peter Sculco, MD
Geoffrey Westrich, MD
Carl Imhauser, PhD
Shady Elmasry, PhD
Status: Patents Pending
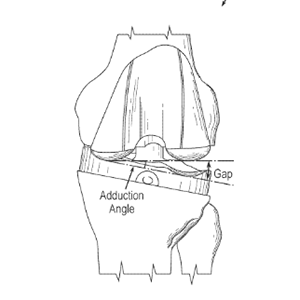
Clinical Problem: In total knee arthroplasty (TKA), surgeons typically apply intraoperative gap assessment to inspect knee balance and soft tissue tensioning after implant trials are placed. Gap assessment is a principal way for surgeons to determine an appropriate tibial insert size and to release ligaments or modify one or more bone cuts in order to achieve proper soft tissue tension. In a typical case, a surgeon performs gap assessment by holding the foot and applying abduction and adduction forces to generate torques at the knee level, while visualizing the medial and lateral gaps at different flexion angles. Unfortunately, this type of examination remains a feel-based, qualitative “art” that relies on the intuition, knowledge, and preferences of each individual surgeon.
Although tools are available to quantify gap assessment, such tools have various limitations, such as by focusing only on resulting medial and lateral gaps.
Solution: System and method to quantify the applied forces/torques during the intraoperative gap assessment exams and subsequent surgical decisions.
Application: This system is a stand-alone product that can be integrated into any TKA procedure.
Arthroscopic hip knife with telescoping blade and guard with adjustable pressure control and handle
Inventor:
William Rodriguez
Status: Patents Granted
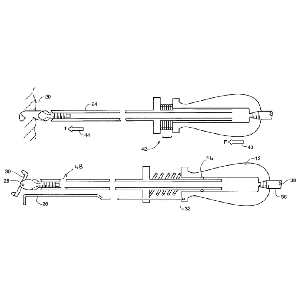
Clinical Problem: In arthroscopic hip surgery, there is a need to provide feedback to the surgeon about anatomic position in the absence of direct visual and tactile feedback. The current invention improves the haptic feedback of arthroscopic surgical tools.
Solution: The device features a telescopic shaft which is attached to a handle on the distal end and a blade coupler on the proximal end. There is a spring between the handgrip and the blade, whereby when force is supplied to compress the spring, there is visual feedback in the form of a displacement guide in the hand grip.
Application: This invention can be incorporated into any surgical kit.
Patient-specific tibial cutting guides for unicompartmental knee replacement to ensure correct orientation of the component
Inventor:
Geoffrey H. Westrich, MD
Joseph Lipman, MS
Status: Patents Granted
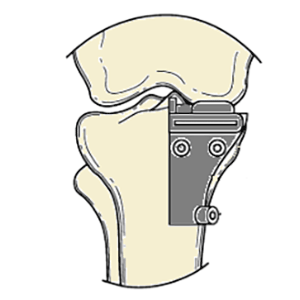
Clinical Problem: Many of the early revisions of unicompartmental knee arthroplasty (UKA) seem to be due to implant-related problems, such as malpositioning, and this may at least in some cases relate to the experience level of the operating surgeon. Thus, an easy and affordable approach for reproducible implantation of UKA components would be highly desirable.
Solution: Patient-specific tibial cutting guides for unicompartmental knee replacement to ensure correct orientation of the component
Application: This system can be integrated into an existing knee arthroplasty portfolio.
Device for removal of bacteria and biofilms from surfaces of implantable medical devices as well as from the surgical site.
Inventor:
Alberto Carli, MD
Kelly Stelmaszczyk
Status: Patent Pending
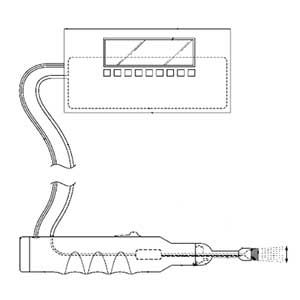
Clinical Problem: Surgical site infection (SSI) is a devastating complication that can occur following urgent or elective surgery, often requiring the need for morbid revision surgery to occur. A principle reason for why infections can persist at surgical sites is the ability for pathogens (e.g., bacteria and/or fungi) to adhere to medical devices, implants, meshes and sutures, and then form a biofilm that permits pathogens to proliferate while protecting them from the immune system, antimicrobial medications and direct surgical irrigation.
Effectively disrupting biofilm, killing microorganisms and leaving any survivors in a planktonic (non-biofilm) state which renders them vulnerable to the immune system and antimicrobials, is known to be the fundamental process required to cure SSI.
When treating SSI, surgeons currently have inadequate tools which cannot effectively mechanically dislodge and disrupt biofilm that has formed on the surfaces of medical implants and adjacent host tissues. Surgeons can currently: (1) irrigate the surfaces and/or (2) submerge the wound in antiseptic solutions of varying concentrations and/or (3) use fragile sponges or toilet-brush-type devices to mechanically disturb biofilm.
Solution: A powered hand-held device for cleaning bacteria and biofilm from a surface. The device offers varying oscillation and sonication designed for different contact surfaces - delicate tissue (skin, subcutaneous tissue), firm tissue (scarred subcutaneous tissue, muscle, bone) and implant surfaces.
Application: This system is a stand-alone product that can be integrated into a PJI treatment portfolio.
Inventor:
Tazeem Buz
Status: Patent Granted
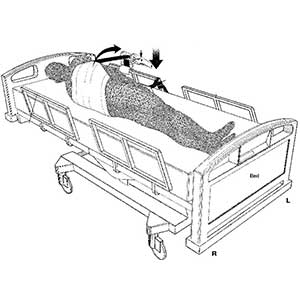
Clinical Problem: Traditionally, turning a patient and changing his or her sheets is a two-person job, as the patient must not only be turned onto his or her side, but also held there while the patient is cleaned (e.g., bathed, etc.) and their sheets are changed. By requiring two people to perform this task, valuable resources are potentially wasted and there is therefore a need for an improved system that permits a single health-care to easily and efficiently perform this entire task, with no discomfort to the patient.
Solution: A strap-and-ratchet system designed for deployment on and under hospital beds, the operation of which will enable a single health-care worker to easily, safely, and efficiently turn a patient onto either side and hold the patient there, comfortably, while the health-care worker cleans and tends to them and changes their sheets and then turns and lowers the patient once more onto their back.
Application: This system can be a permanent feature of hospital beds (i.e., the ratchet device can be permanently mounted to the bed frame), or the system can be deployed with any existing bed on an as-need basis.
Device designed to standardize and directly control contact force at the transducer-patient interface
Inventor:
Daniel R. Sturnick, MS
Status: Patent Pending
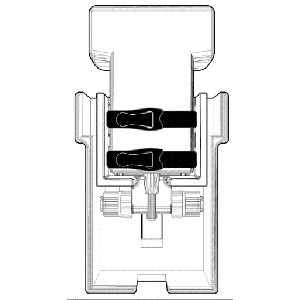
Clinical Problem: An ultrasound protocol growing in popularity is shear wave elasticity, which can assess the mechanical properties of tissue (e.g. stiffness) and has been used in the diagnosis of liver fibrosis, breast cancer, and, recently, various musculoskeletal tissue pathologies. Outcomes of ultrasound diagnostics, particularly evident in shear wave elastography, are sensitive to the applied force between the sonographer and the subject. This leads to variability within an exam and difficulty in reproducing the same results in a subsequent exam, particularly if performed by a different sonographer. For example, in liver fibrosis detection, the apparent morphology and stiffness outcomes from shear wave imaging of the fibrosis are affected by the pressure imparted by the operator on the probe and could lead to a misdiagnosis or a false positive.
Solution: The constant force probe handle is a device designed to standardize and directly control contact force at the transducer-patient interface. The handle allows the transducer head to displace along a low-friction path separately from the operator’s hand position. The handle permits control of a wide range of applied forces by use of interchangeable constant force springs with various specifications, allowing the clinical use case to dictate what target force control is required.
Application: The CF Probe can be integrated with any ultrasound system.
Cost-effective, patient positioning table and techniques that is capable of supporting a patient in one or more medically relevant positions
Inventor:
Helene Pavlov, MD
Roger Widmann, MD
Howard Hillstrom, PhD
Andrew Kraszewski, PhD
Arkady Blyakher
Mary Campolongo
John Denneen
Mark Lenhoff
Edward White
Status: Patents Granted
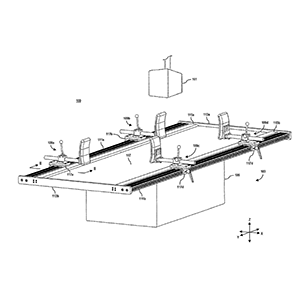
Clinical Problem: Assessment of curve flexibility (or elasticity) is a critical step in determining the structural nature of a deformity, as well as in planning for surgical correction. For instance, idiopathic scoliotic curves are often evaluated, diagnosed, and treated based, at least, on information learned from one or more radiographic images obtained when maximum feasible traction is applied to the idiopathic scoliotic curves without harming a subject (or patient). These radiographic images typically include multiple “bending films” that are exposed while the structural deformity is flexed or otherwise bent. As such, acquiring a bending film requires a patient, or a selected portion of the patient's body, to be forcibly positioned and, thereby, constrained in one or more orientations during radiographic imaging.
Unfortunately, conventional patient tables have inadequately met the needs of arranging and supporting a patient in certain medically relevant positions, such as multipoint bending positions. For instance, conventional patient tables require one or more administrative assistants, doctors, technicians, or other personnel to physically arrange and hold the patient in a required multipoint bending position while the radiographic images are acquired, which exposes these individuals to unnecessary radiographic radiation.
Solution: Cost-effective, patient positioning table and techniques that is capable of supporting a patient in one or more medically relevant positions
Application: This system is a stand-alone product that can be integrated into any clinical or research setting.
Apparatus and method to evaluate the patella in its functional position by acquiring a functional radiographic view equivalent to the standard supine merchant view of the patellofemoral joint.
Inventor:
Fernando J. Quevedo Gonzalez, PhD
David Mayman, MD
Ralph Lopez
Yolanda Wojcik
Status: Patent Pending
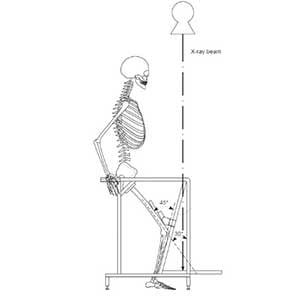
Clinical Problem: Conventional merchant radiographic views allow surgeons to assess the status of the patellofemoral joint. This radiographic view creates a horizon view of the patellofemoral joint that shows the patellofemoral joint space, allowing a physician to measure relevant radiographic parameters, like patellar tilt, patellar subluxation, or patellofemoral contact. To acquire this standardized radiographic view, patients lay supine, while their knees are positioned in 45⁰ flexion. During acquisition of this supine merchant view, the extensor mechanism of the knee (i.e., the quadriceps muscle) remains relaxed.
However, during function of the knee, the extensor mechanism activates to allow knee motion which affects the position of the patella relative to the trochlear groove. Therefore, the position of the patella within the trochlear groove in supine merchant views may differ from that during daily activities, therefore hampering appropriate functional evaluation of the patella.
As such, a need remains to allow evaluation of the patella in its functional position.
Solution: An apparatus and method to evaluate the patella in its functional position by acquiring a functional radiographic view equivalent to the standard supine merchant view of the patellofemoral joint where the extensor mechanism of the knee is active. Activation of the extensor mechanism of the knee can be achieved with a weight-bearing merchant view with the patient in a squatting position. The apparatus includes a positioning device that ensures approximately 45⁰ knee flexion and approximately 30⁰ between the femur and the X-ray beam.
Application: This system is a stand-alone product that can be integrated into any clinical or research setting.
System for securing and loading a knee joint for reproducible magnetic resonance imaging (MRI) for analysis of cartilage deformation caused by the load.
Inventor:
Suzanne Maher, PhD
Scott A. Rodeo, MD
Russell F. Warren, MD
Hollis Potter, MD
Matthew F. Koff, PhD
Hongsheng Wang
Status: Patent Granted
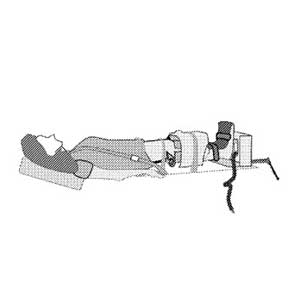
Clinical Problem: The standard of care for providing a full diagnosis of joint injury or degenerative changes is unloaded MRI scanning. However, information can be gathered about tissue deformation under load in a pre-operatively may provide complimentary information for surgical planning. This information would help the clinician to identify the abnormal joint contact, and therefore to choose an appropriate surgical technique (use of scaffolds/number of sutures/use of biological augments, amount of tissue resection) to improve the surgical outcomes.
Solution: An MRI-compatible system for securing a subject's knee joint and generally maintaining a known mechanical load on the knee joint. The system is capable of setting and generally maintaining a mechanical load on the knee joint of a subject while the subject receives an MRI scan inside a main magnet of an MRI scanner. In addition to positioning the subject's knee joint securely with repeatability in patient-specific knee joint orientation for the MRI scan, the system is also capable of delivering patient-specific load to the subject's knee such that the subject's knee joint can be scanned at different time points when the knee joint is under generally the same patient-specific mechanical load.
The system may enable longitudinal studies of knee joints to track cartilage degeneration as well as cartilage response to surgical intervention and/or drug therapies. As MRI scans of the knee joints can be performed with reproducibility when the knee joint is under a load condition, subsequent analysis can quantify features of, for example, cartilage deformation caused by the load. Such ability is advantageous to aid the understanding of the mechanical pathway to joint degeneration, either with or without surgery.
Application: This system is a stand-alone product that can be integrated into any existing MRI platform.
Device that allows for simplified application and the ability to maintain joint motion
Inventor:
Robert Hotchkiss, MD
Joseph Lipman, MS
Status: Patent Granted
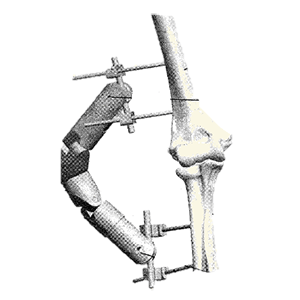
Clinical Problem: Current external fixation technology includes static and hinged fixation systems. A drawback with static systems is that the joint becomes stiff without motion. It can also be difficult to dismantle and reassemble for post-operative mobilization. Hinged devices are challenging to apply and, unless used on a frequent basis, can require long operative time. The complexity of these devices had limited widespread adoption, resulting in inadequate treatment and poor outcomes.
Solution: A simplified device to maintain joint motion and can be easily locked in an original position and then unlocked during rehabilitation. Following a rehabilitation episode, the device can be relocked to stabilize the joint and permit healing.
Application: This system is a stand-alone product that can be integrated into any rehabilitation program.
Inventor:
Sheeraz Qureshi, MD
Harvinder Sandhu, MD
Sohrab Virk, MD
Status: Patent Pending
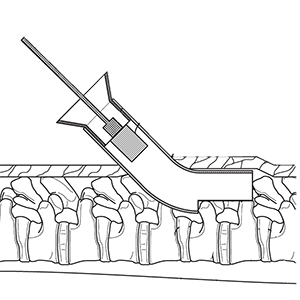
Clinical Problem: Traditional spinal fusions typically involve a large open dissection with pedicle screw fixation. This technique is associated with muscle damage, risk of infection, extended surgical times, long hospital stays and possible injury to sensitive neural elements. Recently, there has been an increasing emphasis in spinal surgery for muscle-sparing procedures that utilize sophisticated techniques/instruments to achieve the goals of surgery in a minimally invasive manner and assist with rapid recovery from surgery that addresses risks associated with traditional spinal fusions.
Solution: System of curved trocars, flexible cannulas and rasps, and bone graft delivery container, and method to percutaneously fuse adjacent vertebrae
Application: System and method can be used for other applications to add stability to the construct and reduce concerns regarding pseudarthrosis, e.g., as a stand-alone technique for spinal fusion, a technique for minimally invasive revision spinal fusion, or combined to augment fusion with other forms of MIS fusion surgeries such as ALIF, TLIF, LLIF, and interspinous fusion. Further, the percutaneous method may be used to augment bony healing for bone fractures (e.g., femur, humerus, tibia, etc.).
This system is a stand-alone product line that could accompany a biologic or MIS system.
A novel system of ultrasound-based referencing of spinal landmarks coupled with a conventional camera-based navigation system or an augmented reality system.
Inventor:
Frank Schwab, MD
Frank Kosarek, MD
Virginie Lafage, PhD
Status: Patent Pending
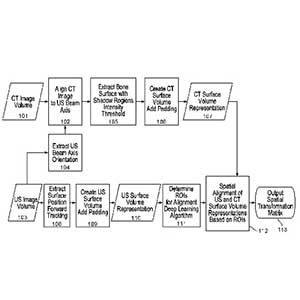
Clinical Problem: The past decades have witnessed great strides of intra-operative imaging systems to enhance the visibility of structures within the patient. Navigation systems have also evolved to provide the surgeon with guidance of the safe trajectories for surgical procedures. However, these systems typically require either fine-cut CT scans pre- operatively in addition to intra-operative fluoroscopic images or intra-operatively acquired fluoroscopic CT scans. These acquisition modes can expose patients and, in some instances, medical professionals to extensive radiation doses. Additionally, during the intra-operative procedure, a reference marker affixed to the patient is displaced, thereby opening the gate to a moving target. In such a circumstance, additional radiographic images can markedly slow down the procedural flow and risk the contamination of the surgical field.
Solution: A novel system of ultrasound-based referencing of spinal landmarks coupled with a conventional camera-based navigation system or an augmented reality system.
The advantages of the system include intraoperative use of ultrasound probes to map and match surface topography in relation to segmented pre-operative imaging (CT, MRI). The advantages of this system also include radiation-free intra operative navigation, improved acquisition speed of surface topography, decreased burden of workflow, and reduced infection risk.
Application: This system is a stand-alone product or can be integrated into a current navigation system.
Coupling anchors for compression of bones via a “zip-tie” or ratcheting mechanism
Inventor:
Steve K. Lee, MD
Andrew Liszewski
Status: Patent Pending
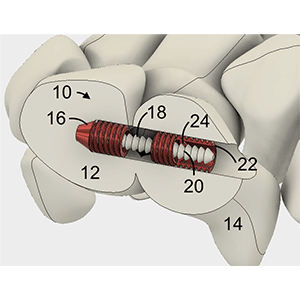
Clinical Problem: Joints such as the wrist and ankle have complex bone and ligamentous structures. Ruptures of the scapholunate ligament of the wrist are common and often require the coupling of the lunate and scaphoid carpal bones to be restored in order to restore natural joint motion and stability. Current procedures involve complex techniques, such as using an autograft transplant to replace the ligament between these two carpal bones.
Solution: A coupling device for joining two bones or bone segments with a tether by placing an anchor in each bone and threading the tether from one through the other; obtaining tension in the tether (compressing the bones) via a "zip-tie" or ratcheting type mechanism.
Application: The device was designed to bridge the scaphoid and lunate bones for scapholunate tears but could also be applied to other small bones in the hand, foot, ankle or spine. Examples include syndesmosis in the wrist and ankle, AC joint in shoulder, basal joint in the thumb, and the facet joint in spine.
This system is a stand-alone product line that can be integrated into an extremity or spine portfolio.
Novel patch design and instrumentation for the augmentation of healing with the addition of PRP or stem cells to the patch
Inventor:
Struan H. Coleman, MD, PhD
Status: Patents Granted, Patents Pending
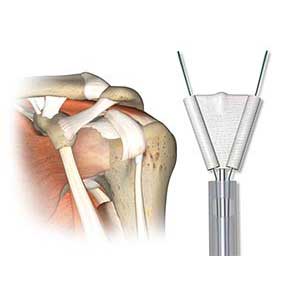
Clinical Problem: Approximately 250,000 rotator cuff repair procedures are performed each year to alleviate the persistent pain and discomfort associated with shoulder injuries and help patients regain full range of motion. There is a significant need for improved use and integration of devices to aid in and augment healing of a repair within an existing surgical workflow.
Solution: Different types, constructs, and methods of suture delivery surgical repair patches and corresponding instrumentation to repair and reconstruct torn ligaments and tendons in a variety of locations within the body. This novel patch design provides for augmentation of healing with the addition of platelet-rich plasma (PRP) or stems cells.
Application: Any surgical repair of a damaged tendon to bone, either performed arthroscopically or utilizing an open technique. This system is a stand-alone product line that can be integrated into a sports medicine portfolio.
Inventor:
Riley Williams, MD
William Rodriguez
Status: Patents Granted
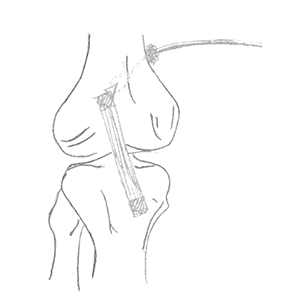
Clinical Problem: There is a need for bone-based fixation of soft tissue structures in many common Sports Medicine procedures, including repair or reconstruction of the ACL, meniscus, rotator cuff and labrum. Conventional fixation devices such as suture screws, anchors, buttons, and pins, are made of metal. Such metal anchors add additional costs to the surgery and are visible to patients on x-rays. Any ill-placement or dissociation of these anchors would also be apparent on films.
Solution: OrthoKnot is a deployable all-suture alternative to existing suture fixation mechanisms. OrthoKnot uses only a suture fiber composed of a series of knots which can be knotted together to form a cluster of knots that forms the anchor to the bone. It offers a more efficient and cost-effective solution to the existing anchoring techniques. As an all-suture device, it is not visible on imaging. As the mechanism for fixating to bone is the suture itself, there is a reduced risk of the anchor becoming separated or dislodged from the suture post-operatively.
Application: OrthoKnot can be integrated into any existing suture line.
Bone anchor, featuring a hollow interior and large graft windows, for coupling and reversible fine-tuning of graft tension
Inventor:
J. Albert Diaz, MD
Status: Patents Pending
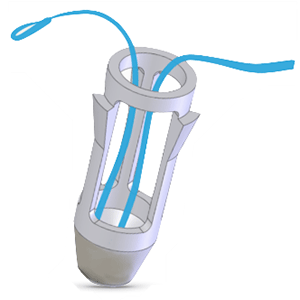
Clinical Problem: There remains a need for devices and methods that overcome the shortfalls of conventional techniques and allow surgeons to efficiently and reliably affix and tension tissue within a bony socket.
Solution: Tenodock is a novel implant that simplifies tenodesis and ligament reconstruction that decreases operative time and eliminates unnecessary radiation exposure. It offers fast, satisfying, soft tissue fixation within a bony socket with the additional benefit of reversible fine-tuning of graft tension.
Application: Potential clinical applications include ligament reconstruction, tendon transfers in the shoulder, knee, foot and ankle, hand, wrist and elbow. Specific indications include, but not limited to, long head biceps tenodesis proximal biceps tenodesis, MPFL reconstruction, multi-ligament knee reconstruction (to include ALL), FHL transfer, UCL reconstruction, and biologic ligament bracing/augmentation with palmaris longus.
This system is a stand-alone product line that can be integrated into a soft tissue fixation portfolio.

A computerized system and method to assess joint level biomechanics and fixation level biomechanics of joint arthroplasty devices.
Inventor:
Fernando J. Quevedo Gonzalez, PhD
Jonathan Glenday
Joseph Lipman, MS
Timothy Wright, PhD
Jonathan Vigdorchik, MD
Peter Sculco, MD
David Mayman, MD
Cynthia Kahlenberg, MD
Status: Patent Pending

Clinical Problem: Although known approaches, such as musculoskeletal multibody dynamics models and finite element models, provide detailed information regarding joint level and fixation level mechanics in total knee arthroplasty procedures, there remains a disconnect between those levels in biomechanical analyses, which hinders an understanding of total knee arthroplasty functionality as a whole.
For example, detailed biomechanical analysis of aspects of total knee arthroplasty biomechanics, such as computational studies, often focus either on the mechanics at the joint level (e.g., joint kinematics and joint loads) or at the fixation level (e.g., bone-implant interaction). This gives rise to the disconnect between joint level mechanics and interface level mechanics, as well as to identifying tradeoffs there-between.
Solution: Joint biomechanics can be assessed simultaneously at the joint and fixation levels via a computerized simulation environment, for example, that integrates the outputs from musculoskeletal models and coupled with finite element models. The combination can be used to determine the choice of the implant, for example, its position, orientation, constraint, and/or design for optimal joint and fixation mechanics. Information can be generated and used for a patient-specific surgical plan, including for the component design, position, and soft tissue balance to improve the longevity and function of an implant.
Application: This system is a stand-alone product that can be integrated into a TKA portfolio.
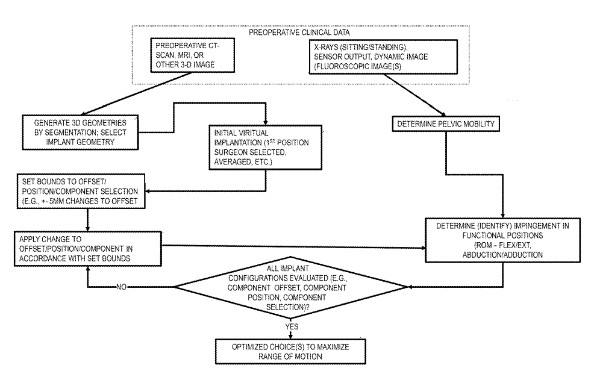
System and method for virtually optimizing implant configurations to maximize range of motion during functional positions in connection with total hip arthroplasty.
Inventor:
Jonathan Vigdorchik, MD
Fernando J. Quevedo Gonzalez, PhD
Eytan Debbi, Md
Joseph Lipman, MS
Timothy Wright, PhD
Status: Patent Pending

Clinical Problem: Dislocation is a common complication following surgical procedures involving total hip arthroplasty (THA) or revision THA, and is a predominant cause of revision and re-revision THA surgery. Impingement, which frequently precedes dislocation, can be characterized as prosthetic-prosthetic, prosthetic-bone, and bone-bone. Prosthetic impingement is a frequently reported type of impingement and is influenced by the position of the components. Bone-bone impingement, although less reported, is also a frequent source of impingement.
Evaluating impingement and, accordingly, the likelihood of dislocation has been difficult to determine. This is at least in part due to an inability to quantify the three-dimensional effect of various component offset changes. While changes to offset are often reported as two-dimensional measures on AP pelvic radiographs, the position of the bones in dislocation-prone positions can be different from their position on AP radiographs. Furthermore, changes to offset often occur in three directions. Consequently, the two-dimensional measurements on AP radiographs may not adequately capture the magnitude of offsets or their effect on the relative positions of the bones in functional positions.
Solution: A computerized simulation that quantifies the effect that THA component offset has on the range of motion to the risk of impingement during functional positions, including the effect of implant orientation and bony orientation.
Application: This system is a stand-alone product that can be integrated into a THA portfolio.
Quantitatively and objectively measures the 3D displacements of the knee joint in response to multidirectional loads in a clinical setting
Inventor:
Carl Imhauser, PhD
Status: Patents Granted
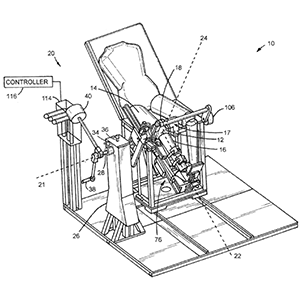
Clinical Problem: Clinical assessment of joint pathologies, including those involving soft tissues or other ligament injuries, is primarily subjective. During clinical examination, the clinician obtains a qualitative and subjective “feel” for the amount of laxity or stiffness in the involved joint as compared to the clinician's previous intuition regarding what the normal joint should feel like and/or compared with the contralateral joint should it be uninjured. Moreover, assessment of the effectiveness of treatment be it conservative (e.g., rehabilitation) or surgical is subjective and relies on feedback from the patient.
Conventional knee arthrometers are unidirectional, measuring the translation or rotation only in the direction of the applied force or torque, respectively. They were not designed to characterize knee rotations and coupled motions occurring in response to multiplanar torques, such as what occurs during the pivot shift exam.
Solution: A quantitative, reliable technique to assess the 3D load displacement response of a joint for the purposes of contributing to the clinical management of joint pathologies.
Application: This system is a stand-alone product that can be integrated into any diagnostic program.
Computer model of a patient specific joint for treatment planning and a method of developing a treatment plan and treating a joint of a patient
Inventor:
Carl Imhauser, PhD
Kevin Schafer
Status: Patent Granted
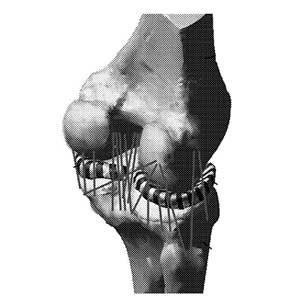
Clinical Problem: Currently there are numerous surgical techniques available to treat joint related issues or injuries of a patient. These techniques are applicable to a wide variety of joints of a patient, such as the knee, ankle, hip, shoulder, elbow, fingers, wrists, and other synovial joints. Such techniques include, but are not limited to, ligament reconstruction and joint replacement.
However, not all joints of individuals of a population function in exactly the same manner and there are large variations in, e.g., morphology, material properties of bones, articulating surfaces and ligaments, in joints between different individuals. Accounting for these variations is an important consideration for planning surgical treatments of such joints. The failure to individually customize surgical parameters based on unique structural and morphological profile of each patient results in suboptimal outcomes.
Quantifying interdependencies among knee kinematics, ligament load, and the influence of implant alignment from patient-to-patient is important. This is currently attempted by computer models of the joint that provide a framework to assess the impact of surgical and patient factors on knee stability and ligament forces. However, conventional computer models of a joint are limited in their ability to predict kinematics and loading patterns in the passive restraints over a large range of flexion. That is, conventional computer models are hindered in their ability to predict kinematic and ligament loading patterns over a large range of flexion because they do not account for variables necessary to properly predict forces and motion between the bones of a joint. In sum, conventional computer models of a joint cannot adequately differentiate the wide range of passive knee stability and ligament loading patterns observed across a population.
Solution: To address the deficiencies of conventional computer models of a joint as well as the conventional deficiencies in planning surgical treatment plans for treating joints, a new method of creating a computer model of a joint that is patient specific has been developed. The new method of creating the computer model of the patient specific joint includes identifying ligaments of a joint of the patient that are under a load at full extension of the joint. The identified ligaments are then incorporated into the computer model of the joint. The computer model of the joint is a multibody dynamic model that includes formulations for ligament fiber architecture that reflects its anatomy.
The model advantageously includes ligaments that have been identified as having a load at full extension and constructed based on a predefined slack length to better predict forces on the joint as well as kinematic motion of the joint bones.
The computer model of the joint is then simulated through a range of motion i.e., equations of motion are applied to the computer model, to predict reaction forces and motion of the joint as it moves through the range of motion.
Application: This system is a stand-alone product that can be integrated into an existing diagnostic portfolio.
Intraoperative system and method to allow joint contact mechanics to be measured at various stages throughout the surgery.
Inventor:
Suzanne Maher, PhD
Scott A. Rodeo, MD
Russell F. Warren, MD
Hongsheng Wang
Mario Lustri
Status: Patent Granted
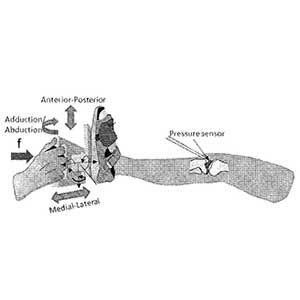
Clinical Problem: The effect of injury on the long-term health of joints, such as the knee, hip and shoulder, has been documented; with most injuries leading to increased risk of the development of osteoarthritis (OA). The long-term goal of surgical treatment is to protect the knee joint from developing OA. Unfortunately, this goal has not been achieved by current surgical methods.
Surgical techniques used to treat injured joints are intended to restore the ability of the joint to mechanically function. For example, mechanical function can be recaptured by restoring stability so that the joint can carry loads in a stable way through the necessary range of motion for daily activities (ACL reconstruction, tendon transfers, rotator cuff repair), or locally repairing the site of damaged tissue so that it can carry or distribute joint loads (articular cartilage repair, meniscal repair).
Assessing patient outcome, however, is difficult. Pain levels and return to daily activities are subjectively scored by the patient in a post-operative environment. Imaging is also used to assess the durability of the repair and the overall status of the joint. But, by the time these measures are made, significant time e.g., 6 weeks to 3 months have passed.
Thus, there is still a need to address the foregoing limitations of conventional surgery.
Solution: A method for intraoperatively measuring joint contact mechanics of a patient's joint is provided. The method includes inserting a sensor between first and second bones of a joint. Then a predetermined force is applied to one of the first and second bones. Afterwards, contact mechanics such as, contact stresses, contact areas and/or forces are measured between the first and second bones in response to the applied predetermined force.
The direct, intraoperative measurement of joint contact mechanics can provide useful information about the mechanical condition of the injured joint, the mechanical condition of the joint during the trial placement of a device intended to replace the joint, the mechanical condition of the joint during a surgery intended to repair an injured tissue, and the mechanical condition of the joint at the end of the surgery.
This information can be used to make adjustments at the time of surgery for implant placement (for example, when assessed at the trial implantation stage of a total knee replacement or the positioning of meniscal allografts), to the tightening of ligaments that span the joint (e.g., when ligament loosening procedures are done during joint replacement), and to give feedback to the surgical team about the mechanical condition of the joint immediately after intervention. Such information may advantageously be predictive of short term or long-term outcome and that the data generated may help identify patients that should be targeted in the primary prevention of osteoarthritis.
Application: This system is a stand-alone product that can be integrated into an existing diagnostic portfolio.
Method of categorizing knees in orthopedics to determine a surgical stratgey
Inventor:
Suzanne Maher, PhD
Russell F. Warren, MD
Scott A. Rodeo, MD
Tony Chen, PhD
Status: Patent Pending
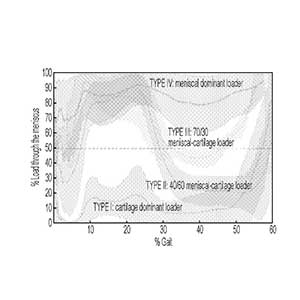
Clinical Problem: The meniscus is a commonly injured portion of a knee or knee joint and is the most operated upon orthopedic tissue, with over 700,000 meniscal surgeries done annually in the U.S. In many cases, repair of the meniscus is not possible, instead, removal of the damaged tissue occurs in a “partial meniscectomy” (PM) procedure. However, removal of damaged tissue causes redistribution of forces across the joint, to which cartilage cannot adapt. As such, many patients show signs of osteoarthritis (OA) within months after surgery.
Current methods to treat injured tissue in the knee joint, for example, include using orthopedic scaffolds to repair or replace an injured meniscus or cartilage in an effort to restore mechanical function however, these are rarely used clinically, in part because it is unclear which patients will benefit most from mechanical reinforcement. Therefore, there remains a need for a method to determine which patients would benefit from scaffolds or other mechanical reinforcements.
Solution: The method includes assigning the knee to a predetermined class based on a determined percentage of load through the meniscus of the knee through a defined range of motion. The method also includes determining a course of medical treatment for the knee based on the predetermined class the knee is assigned.
Application: This system is a stand-alone product that can be integrated into an existing diagnostic portfolio.
Interactive pre-operative patient-specific surgical planning system based on activity-based kinematics, soft tissue balancing, and fixation strength
Inventor:
Andreas Kontaxis, PhD
Lawrence Gulotta, MD
Status: Patent Granted
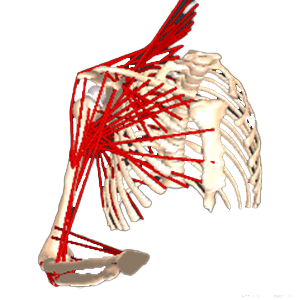
Clinical Problem: In patients that have undergone shoulder replacement surgery, range of motion and strength are dependent on shoulder kinematics, which are in turn dependent on a host of factors such as implant size, implant position, implant design, the joint line and soft tissue tension. Often, a surgeon finds that there are too many variables to manage at one time. Current implant designs and methodologies are inadequate to address these challenges because they are of significant cost, require time to develop, include increased risk of implant failure, and rely on human judgment of potential outcomes post-operatively.
Solution: A computer implemented interactive patient specific surgical planning system for a method of performing a total joint or a partial joint surgery includes: (1) performing a virtual pre-operatively planned joint surgery to implant a prosthetic device; (2) accounting for a range of motion desired for activities of daily living and/or standard clinical assessments of range of motion after performing the virtual surgery; and (3) outputting results for each implant, each location, and each range of motion activity from each virtual surgery performed.
Application: This system can be integrated into existing surgical planning software platforms or can form the basis for a new platform.
Patient specific instruments that facilitate implantation and correct alignment of shoulder implant components
Inventor:
Andrew Pearle, MD
Joseph Lipman, MS
Darrick Lo, MEng
Status: Patents Granted
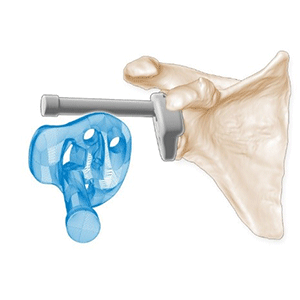
Clinical Problem: Shoulder hemiarthroplasty is commonly used to treat patients with glenohumeral joint arthrosis. For patients with glenohumeral joint arthrosis and an additional deficient rotator cuff, reverse total shoulder arthroplasty may be indicated. The 12%, 15%, and 22% revision rates, respectively, remain high compared to hip and knee arthroplasty. Glenoid component loosening and instability are important complications and may be caused by poor positioning of the components. An accurate placement of the complementary humeral cut is also important to achieve a stable joint. There is a continuing need to improve the instruments used to facilitate the implantation of total shoulder joint components.
Solution: Patient specific instruments that conform to the actual diseased joint surface and incorporate features that facilitate implantation and correct alignment of shoulder implant components. The surgeon uses the instruments to align and direct surgical cuts and to prepare the patient to receive standard or reverse joint implant components.
Application: This system is a stand-alone product line that can be integrated into any shoulder implant system.
An upper extremity prosthesis for use in below the elbow amputations with an osseointegration post
Inventor:
Glenn Garrison
Status: Patents Pending
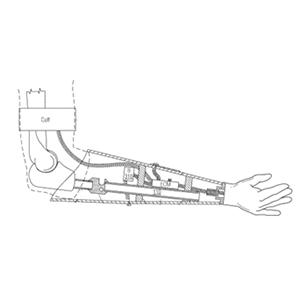
Clinical Problem: A below elbow arm prosthetic is located below the elbow and above the hand and is used after an upper extremity amputation is performed. When dealing with below elbow amputations, the length of the residual limb may cause complications in terms of upper extremity prosthetic fitting, etc.
Solution: An upper extremity prosthesis for use in below the elbow amputations with an improved solution for mounting, especially those with short residual limb lengths below the elbow, and osseointegration post.
Application: This system is a stand-alone product that can be integrated into any rehabilitation program.