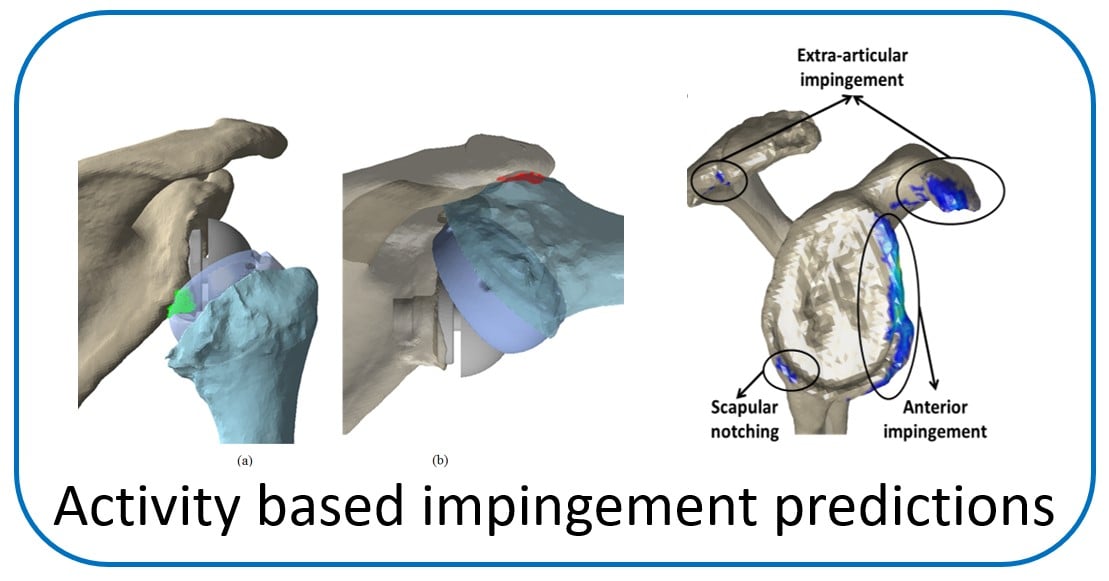
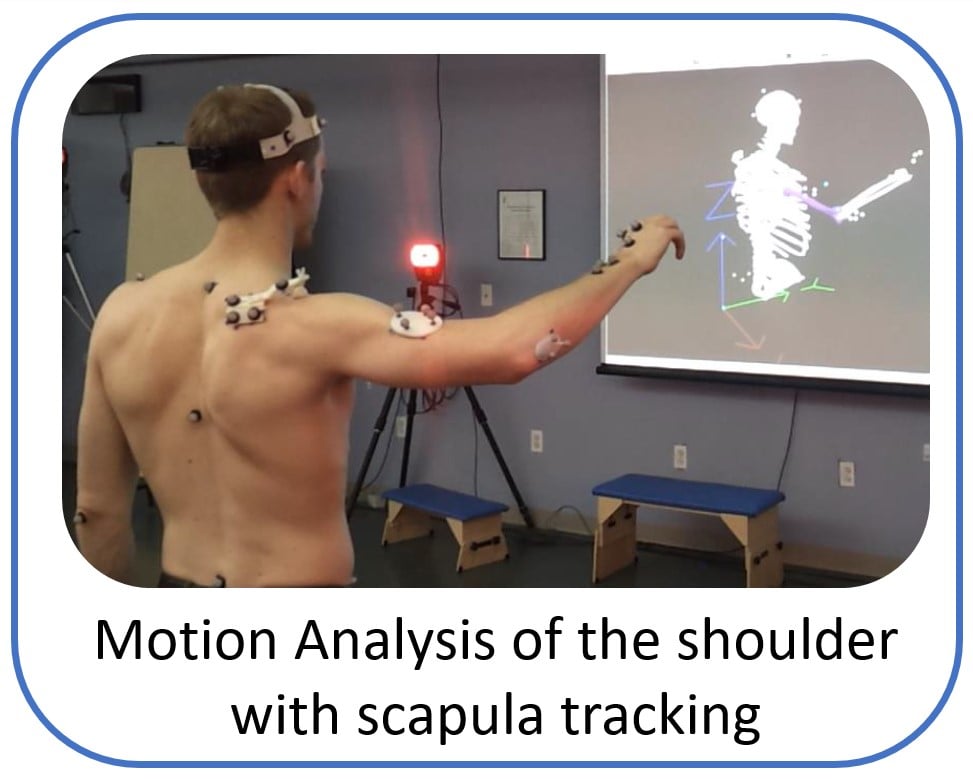
Post Doctoral Fellow
Department of Biomechanics
AMT Center
510 East 73rd Street, Room 202-A
New York, NY 10021
Tel: 646.714.6431
Email: shenoya@hss.edu
About Aarti Shenoy
Dr. Aarti Shenoy joined the Biomechanics department at HSS as a Postdoctoral Fellow in February 2021. Dr. Shenoy is interested in implant and modular junction corrosion and its impact on implant longevity. She has investigated corrosion behavior in hip implants, particularly modular dual mobility designs, using in vitro test methods and retrieval analysis studies in collaboration with the Arthroplasty service. Her research will continue to focus on material science aspects within orthopedic devices: structure-property-processing interactions and their effects on clinical outcomes. Dr. Shenoy is interested in biomedical alloys, their microstructures and how alloy microstructure might affect mechanical properties of the material and the device as well as device performance.
Dr. Shenoy received her PhD in Bioengineering from Clemson University as part of Dr. Jeremy Gilbert’s research group. Her research was focused on test method development to evaluate hip implant mechanical and electrochemical performance, retrieval analysis using microscopy techniques, and studying macrophage-tribocorrosion interactions in vitro. She was a technology transfer and commercialization intern at the Clemson University Research Foundation (CURF) where she acted as a liaison between faculty members developing IP and the tech transfer team, and also developed marketing materials for existing Clemson IP.
Dr. Shenoy also holds a Bachelor of Engineering in Biomedical Engineering from University of Mumbai, India and a Master of Science in Bioengineering from Syracuse University, NY
Awards
New Investigator Recognition Award (NIRA) finalist, 2023
Selected Publications
- C. Kahlenberg, E. Baral, A.A. Shenoy, “Clinical and Biomechanical Characteristics of Posterior-Stabilized Polyethylene Post Fractures in Total Knee Arthroplasty: A Retrieval Analysis”, Journal of Arthroplasty, Feb 2023
- A.A. Shenoy, S.M. Kurtz, J.L. Gilbert, “Non-Tribological Corrosion Modes Dominate Wrought CoCrMo Acetabular Taper Corrosion: A Retrieval Study”, Journal of Biomedical Materials Research Part-B, May 2021 (DOI: 10.1002/jbm.b.34854)
- M. Wiegand, A.A. Shenoy et al., “Sensing Localized Surface Corrosion Damage of CoCrMo Alloys and Modular Tapers of Total Hip Retrievals Using Nearfield Electrochemical Impedance Spectroscopy”, ACS Biomaterials Science and Engineering, Jan 2020 (DOI: 10.1021/acsbiomaterials.9b00945)
- A.A. Shenoy, J.L. Gilbert, “In vitro test methods for seating and fretting corrosion behavior of modular metal-on-metal acetabular tapers”, Journal of Orthopaedic Research, Dec 2019 (DOI: 10.1002/jor.24554)
- E.S. Ouellette, A.A. Shenoy (co-first-author), J.L. Gilbert, “The seating mechanics of head-neck modular tapers in vitro: Load-displacement measurements, moisture, and rate effects”, Journal of Orthopaedic Research, Sept 2018 (DOI: 10.1002/jor.23725)
For more publications, please see the PubMed listing.
Selected Presentations
- A.A. Shenoy, A. Pekmezian, T. Wright, D.E. Padgett, “Corrosion in Modular Dual Mobility Acetabular Components: A Retrieval Analysis Study”, Orthopaedic Research Society Annual Meeting 2023, Dallas TX, USA (Finalist for New Investigator Recognition Award)
- A.A. Shenoy, J. Steele, D.E. Padgett, T.M. Wright, “How do surface finish and taper design influence corrosion modes and mechanically assisted crevice corrosion behavior in modular dual mobility acetabular tapers?”, International Society for Technology in Arthroplasty Annual Meeting 2022, Maui HI, USA (Talk)
- A.A. Shenoy, C Kahlenberg, et al., “Post Fractures in Total Knee Arthroplasty: Differences in Fracture Progression between UHMWPE and XLPE Posts”, International Society for Technology in Arthroplasty Annual Meeting 2022, Maui HI, USA (Talk)
- A.A. Shenoy, J.L. Gilbert, “In Vivo Corrosion of Acetabular Modular Tapers Reduces Local Corrosion Resistance: Near-field Electrochemical Impedance Spectroscopy as an Indicator of the Type and Severity of Corrosion in Retrieved Hip Implants”, Exciting Research in Orthopaedic Implants and How to Get it Funded, Orthopaedic Research Society Implants RIG Section Virtual Session, 2021, (Early-stage career investigators invited talk)
- A.A. Shenoy, J.L. Gilbert, “Near-Field Electrochemical Impedance Spectroscopy Methods for Retrieval Surface Analysis”, International Society for Technology in Arthroplasty Annual Meeting 2019, Toronto, Canada (Talk)
- A.A. Shenoy, S.M. Kurtz, J.L. Gilbert, “Biological, Chemical and Mechanical Damage Modes in Corrosion of CoCrMo Acetabular Liners”, International Society for Technology in Arthroplasty Annual Meeting 2018, London, UK (Young Investigators Session, Talk)
- J.L. Gilbert, A.A. Shenoy, “Bio-metallurgical interactions during in vivo corrosion: Beyond fretting corrosion, a study of the forms of corrosion in retrieved modular implants, their causes and consequences”, ASTM Symposium 2017, Toronto, Canada