Device Development & Innovation
HSS has had a strong device development effort since the 1970s. In addition to designing custom devices for HSS patients, we design standard implant systems that are licensed to orthopedic companies throughout the world.
Working closely with HSS surgeons, we develop implants with the goal of solving real clinical problems. The close integration between surgeons and engineers is invaluable to design excellence and is unmatched at any institution.
With the help of our surgical colleagues, we have developed joint replacements for the hip, knee, ankle, shoulder, elbow, and wrist, as well as devices for enhancing spine fusion and improving spinal stabilization.
In 2021, LimaCorporate opened the ProMade Point-of-Care (PoC) Center, the first provider-based commercial 3D design and printing facility for complex joint replacement solutions at the HSS main campus in New York City. The PoC Center works closely with the Department of Biomechanics for research, product development, and patient care.
Patient-Specific Implants and Planning
We are often asked by our surgeon colleagues to help plan complex cases where it is unclear if off-the-shelf implants will be an appropriate treatment for the patient. We provide 3D reconstruction and visualization, engineering design, patient-specific computational analysis, and 3D printed plastic models to help determine the best treatment option for the patient.
If it is determined that off-the-shelf implants are inadequate, we collaborate with orthopedic device manufacturers from across the industry to provide solutions. Since 1977, we have designed over 2400 patient-specific implants for patients at HSS. General indications for these patient-specific implants include small stature, severe deformity, complex revision, and clinical problems where no known solution exists.
Patient-Specific Flanged Acetabular Component
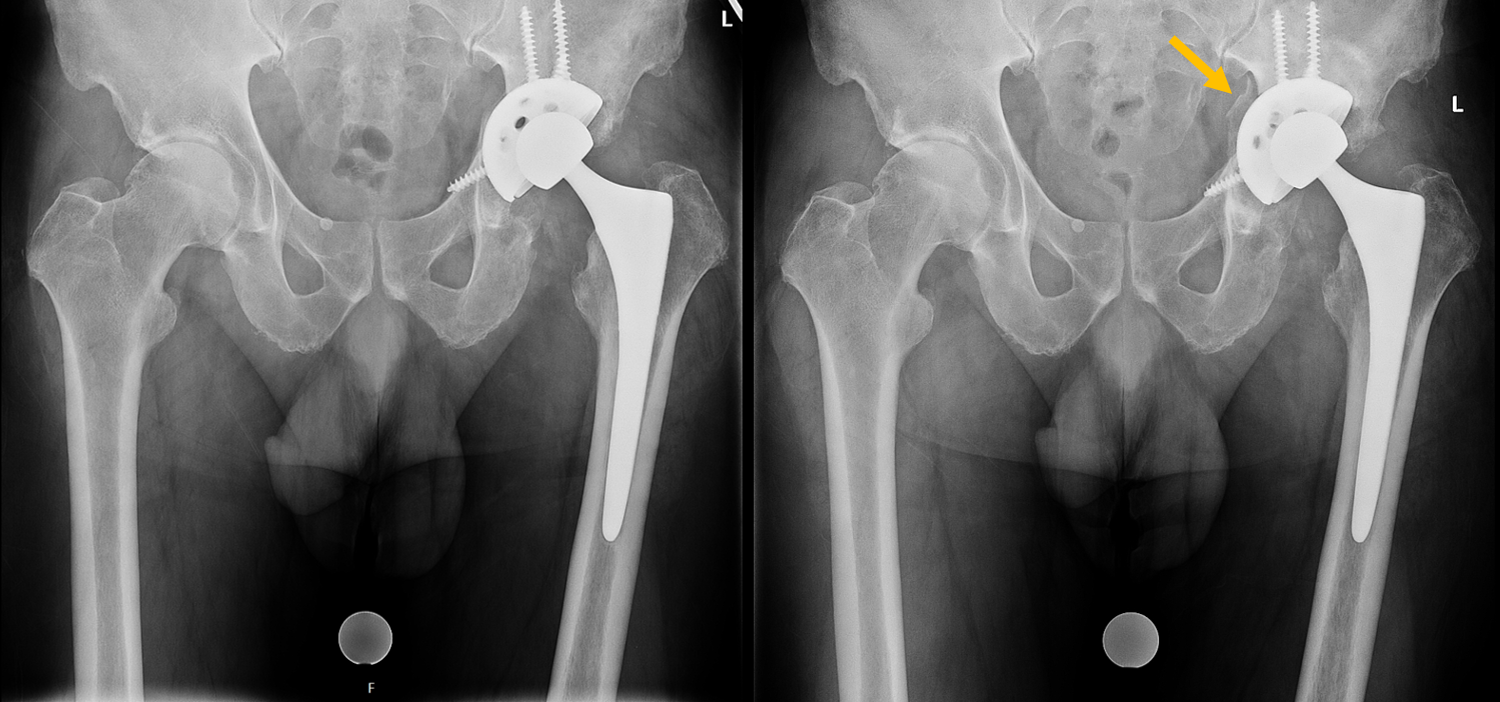
X-rays showing a failed left total hip arthroplasty. There was a fracture of the anterior acetabular column and loosening of the acetabular component. The patient required revision surgery.
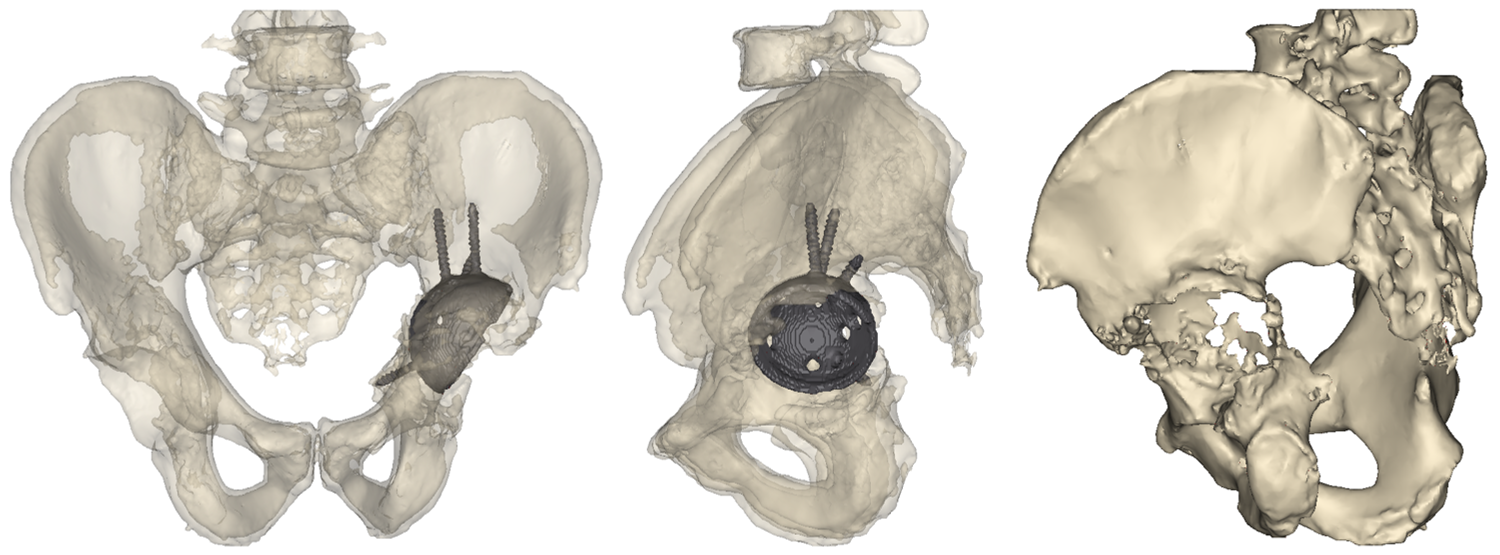
3D reconstruction from CT scan images show the location of indwelling hardware and the extensive acetabular defect.
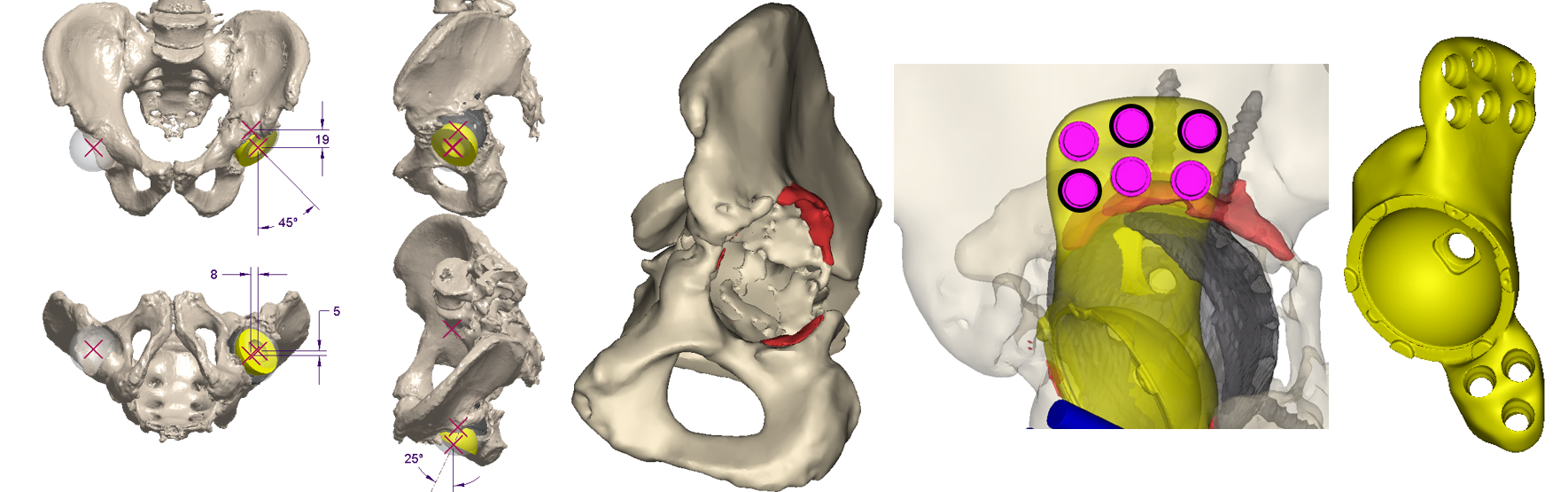
The Device Development team works with the surgeon and outside manufacturers to develop a patient-specific flanged acetabular component to treat the patient’s unique defect.
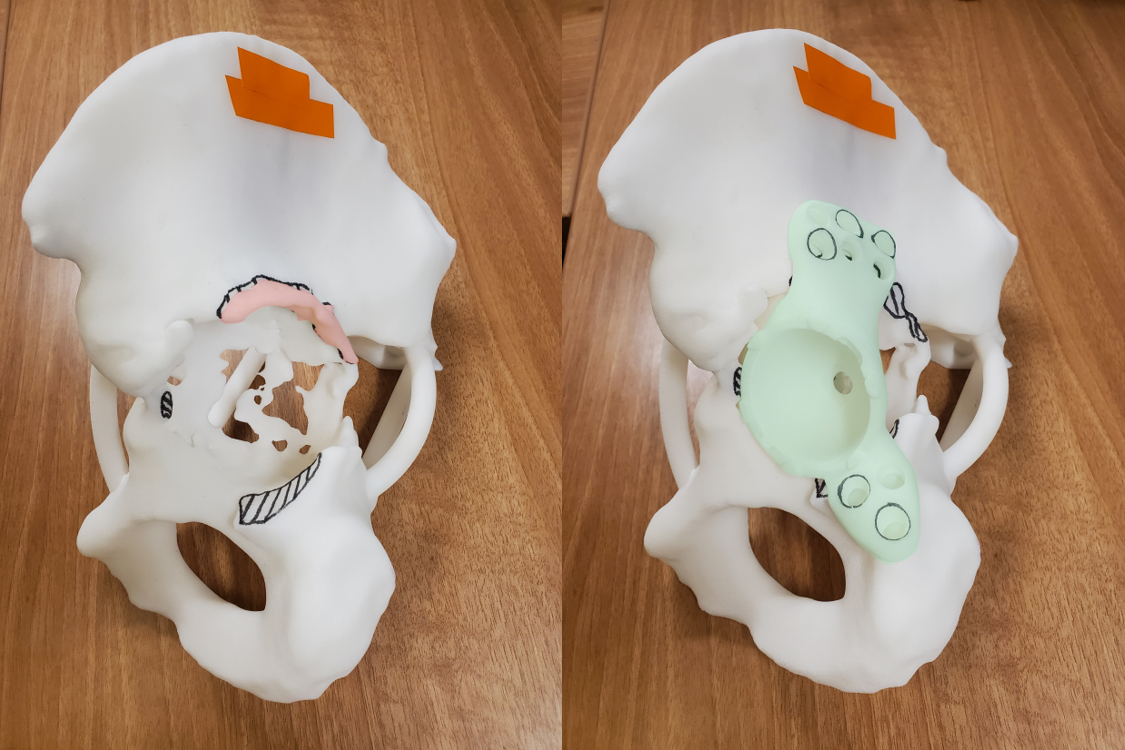
3D-printed plastic models help surgeons visualize and practice the surgical plan.
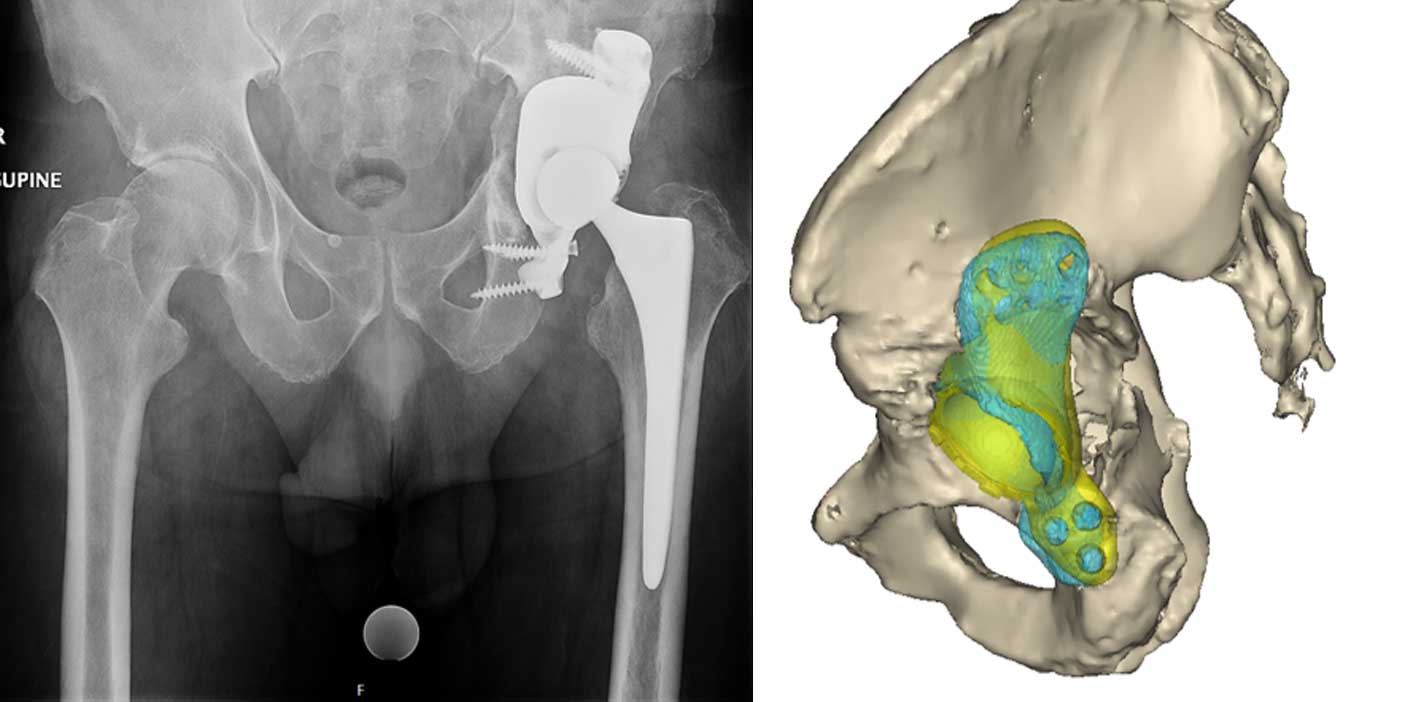
Post-operative images of the patient specific implant. A post-operative CT scan is used to compare the implanted position with the pre-operative plan.
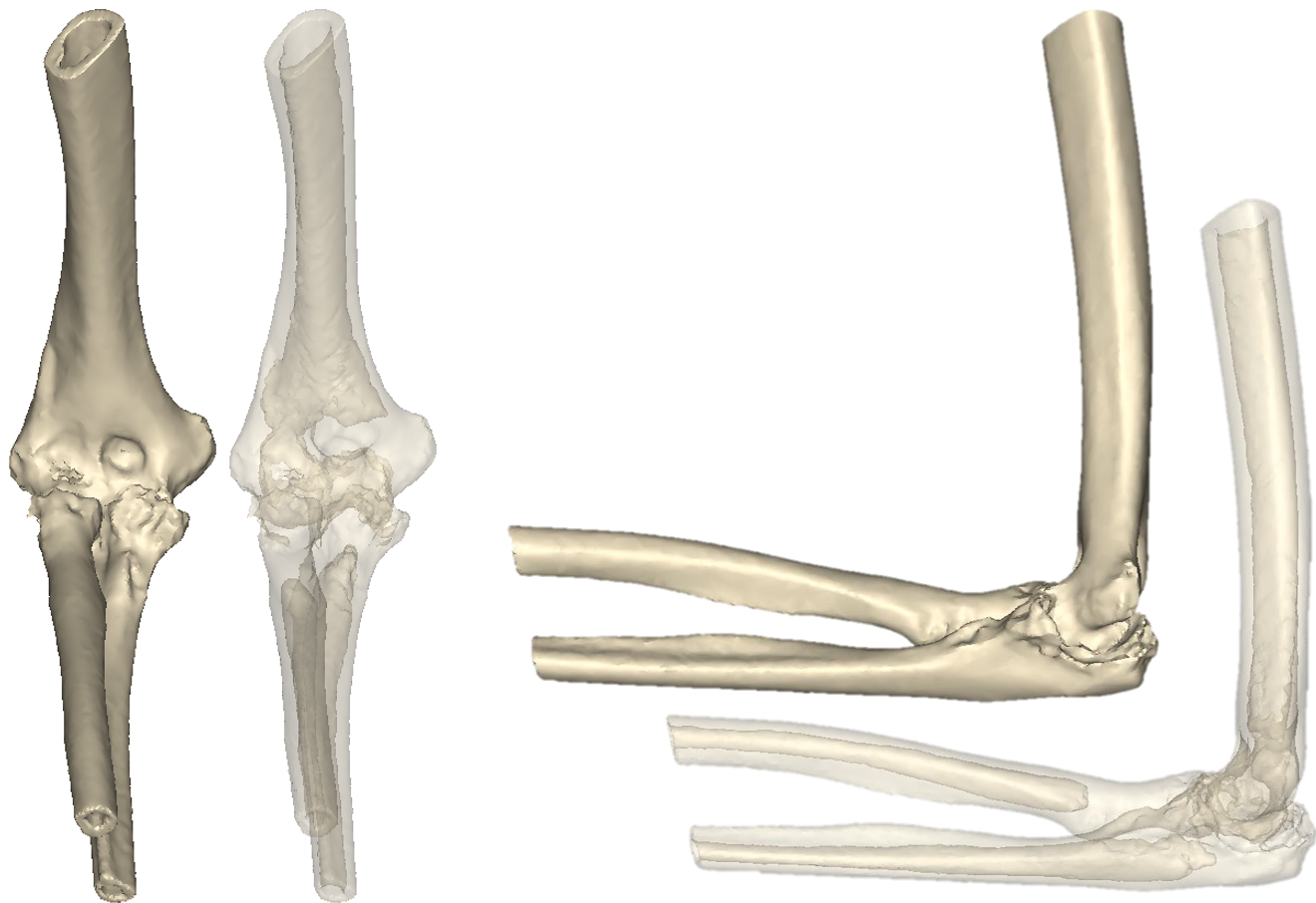
Patient-Specific Total Elbow Replacement

X-rays showing a right elbow of a patient with rheumatoid arthritis. Surgeon would like to perform cementless total elbow arthroplasty (TEA) on the patient.
CT was used for pre-operative planning.
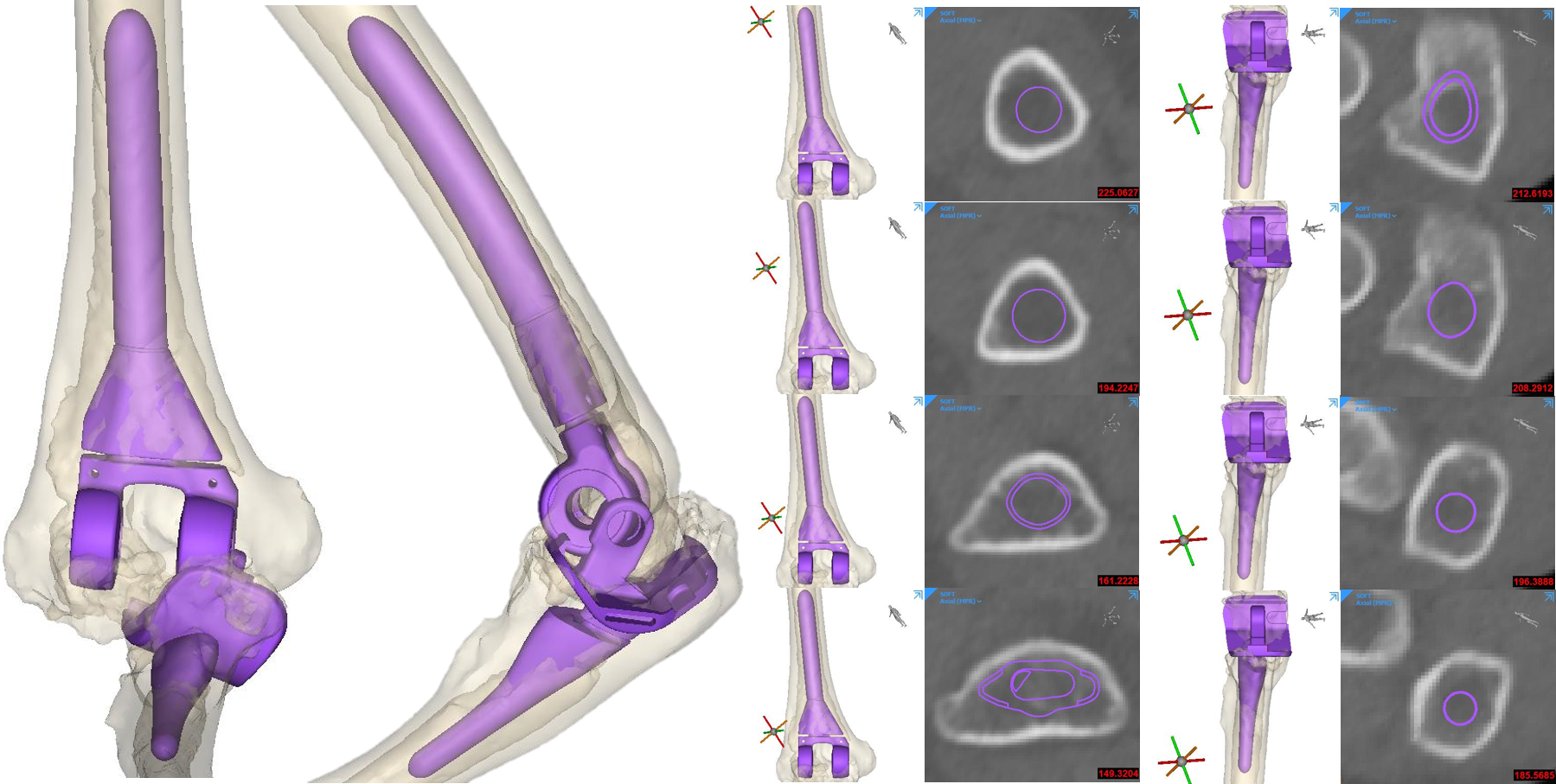
Initial fit check of standard TEA component was done by the device development engineers. Both humeral and ulnar components were too small for a cementless TEA. Patient specific implants were needed.

Device development engineers, surgeons and outside medical device companies collaborated to design the patient specific cementless humeral and ulnar stems.
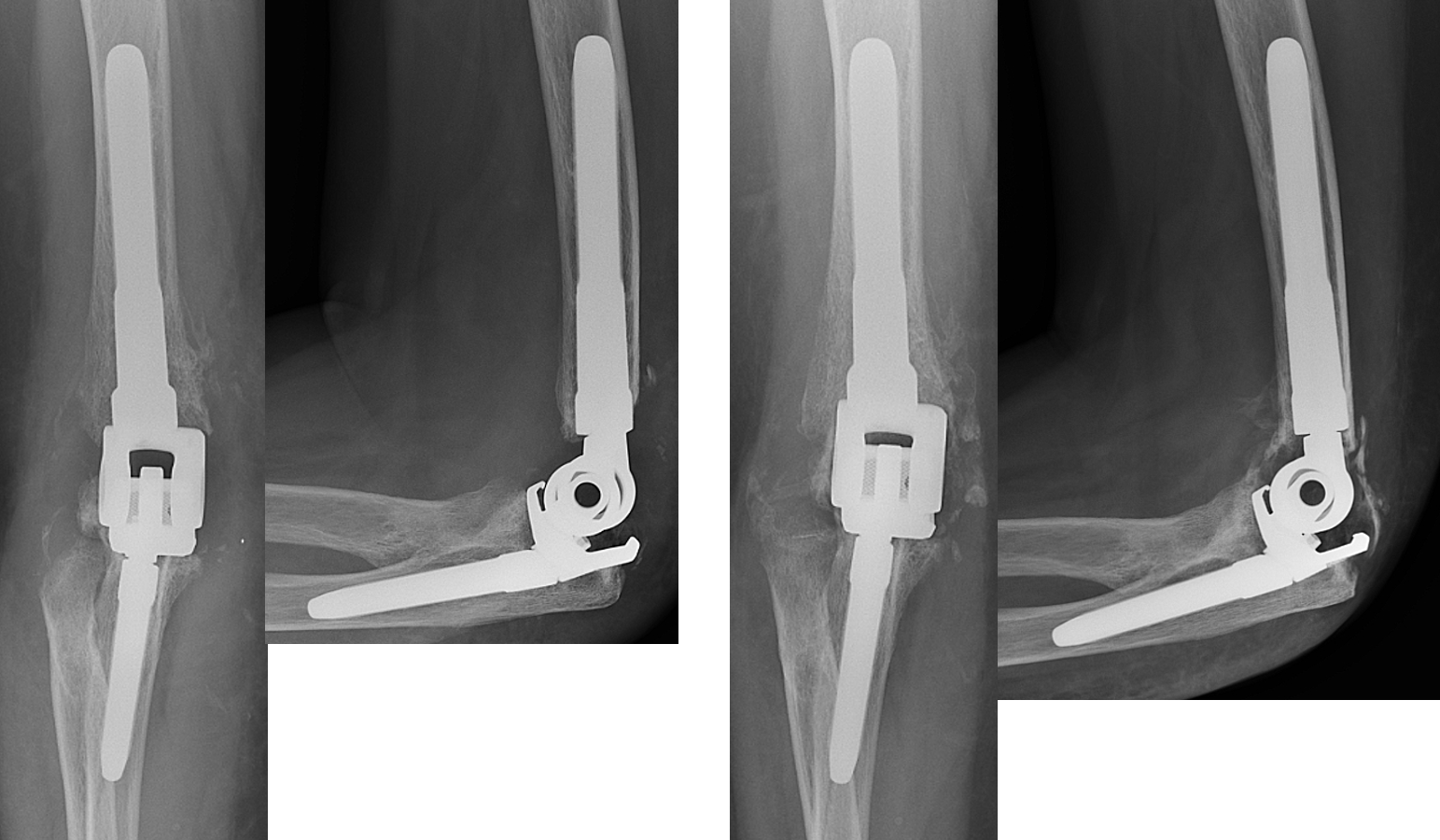
Immediate post-op x-rays (left) and 3-month post-op x-rays (right) showing well-fixed implants and bone ingrowth.
Wrist Planning
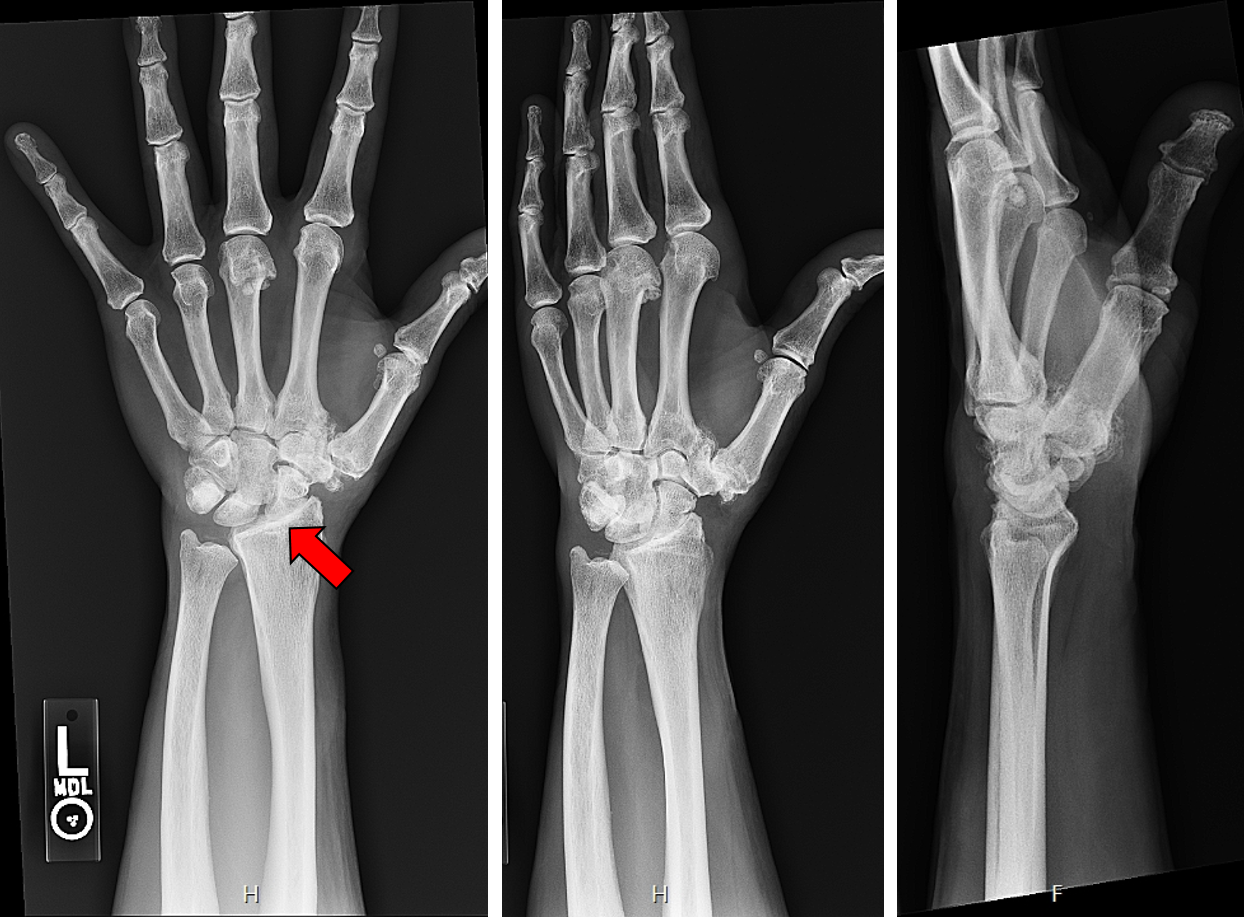
X-rays of a patient with osteoarthritis at the radiocarpal joint resulting in painful bone-on-bone contact. The surgeon would like to perform a total wrist arthroplasty (TWA).
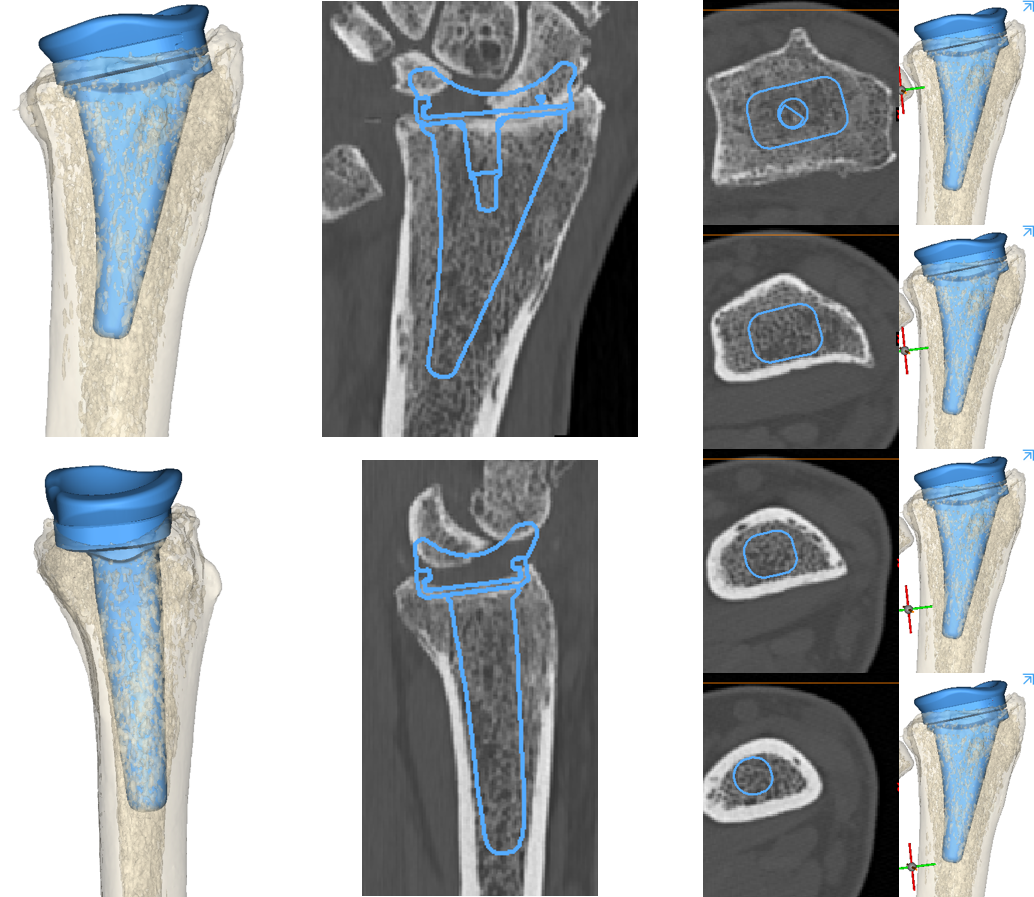
The Device Development team creates 3D reconstructions of the bone to template the size and position of the radial component of the TWA (blue).
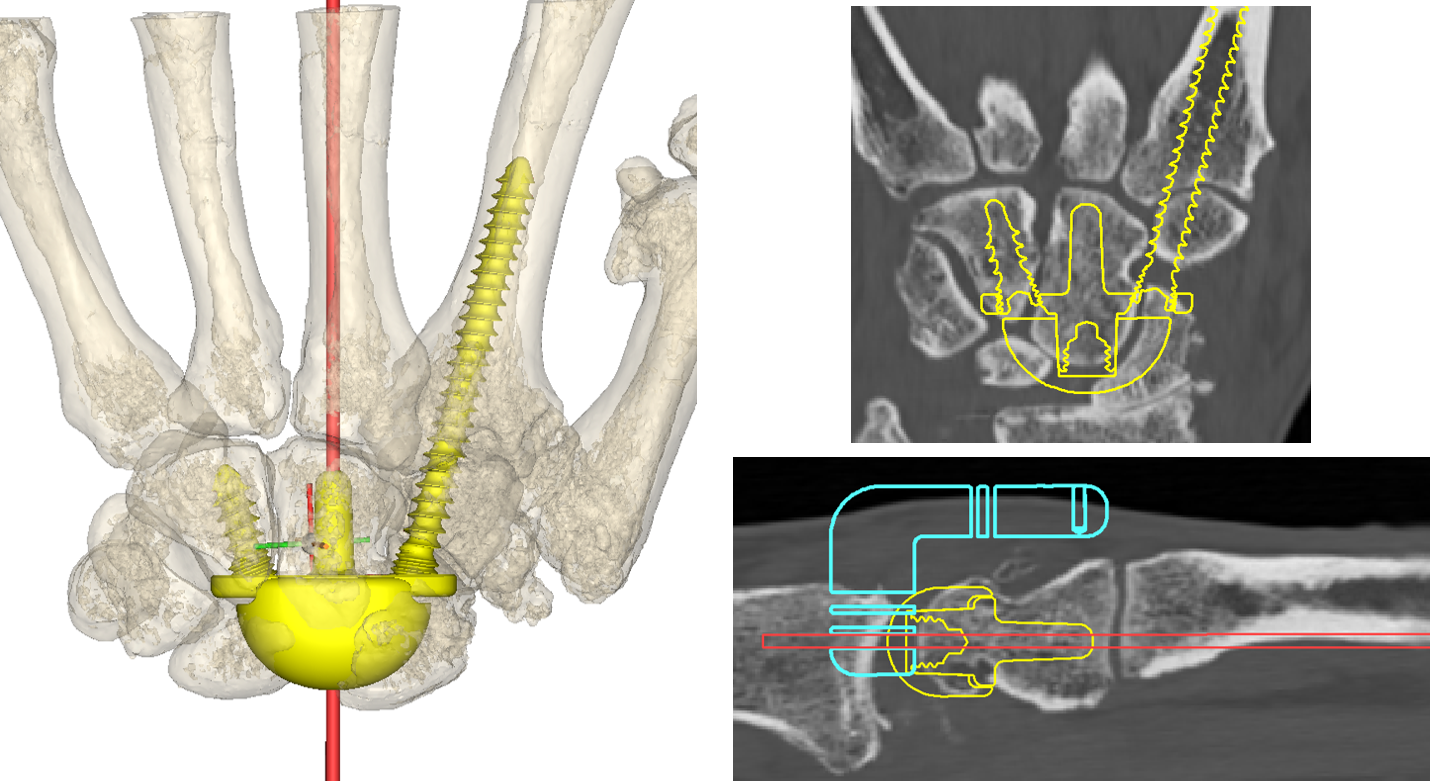
The carpal component of the TWA (yellow) is also templated for size and position.
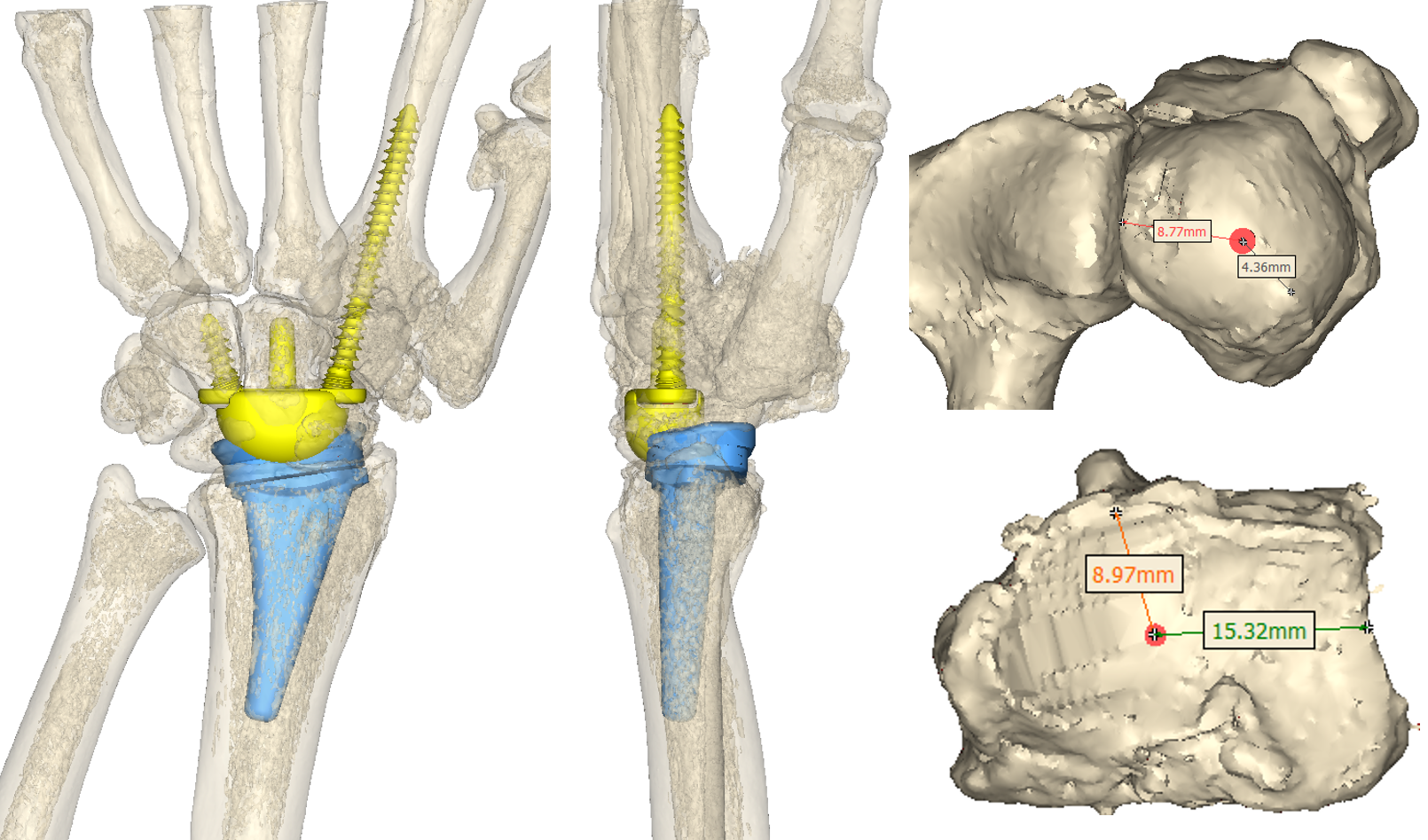
Measurements are provided to the surgeon to assist with accurate placement of guide wires during the surgical procedure.
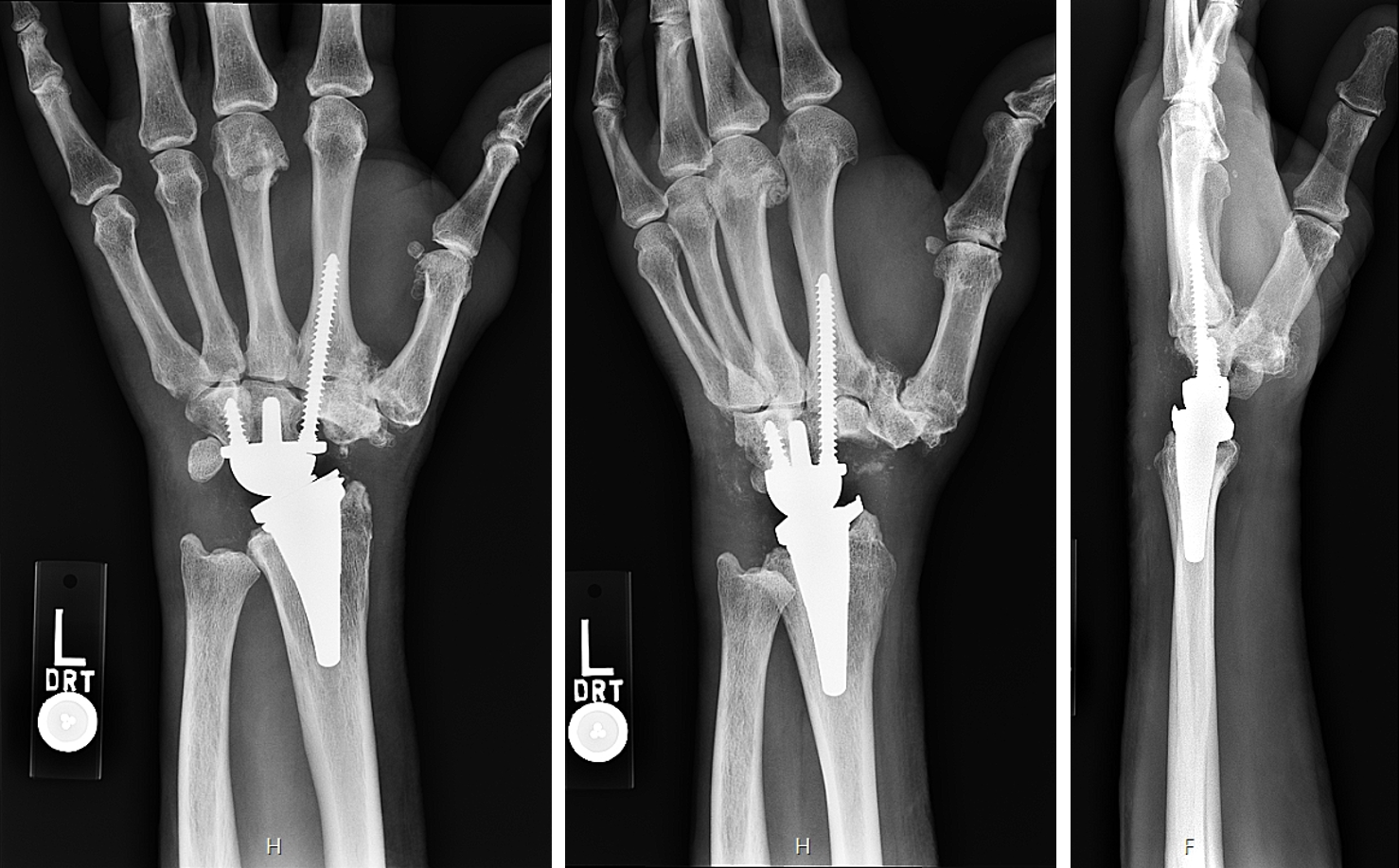
Postoperative X-rays showing the implanted total wrist replacement. Both components match the plan well and appear sized and positioned appropriately.
Product Development
The Department of Biomechanics has a strong history of developing new technology. Close collaboration between surgeons, engineers, and the Innovation Institute and partnerships with medical device manufacturers allow HSS to create new solutions to address clinical challenges. Examples of these collaborations include:
Cementless Tibia Baseplate
Cementless tibia baseplates for total knee arthroplasty require sufficient initial stability at the bone-implant interface to encourage bone ingrowth for long-term fixation. HSS engineers in collaboration with Thomas Sculco, MD and Peter Sculco, MD used patient-specific finite element modeling to improve the fixation features of a 3D-printed cementless tibial baseplate.
Total Elbow Arthroplasty
Clinical review and patterns of damage seen in retrieved implants revealed that early revisions of total elbow arthroplasties were caused by excessive wear of plastic bushings at the hinge. To address this problem, HSS engineers worked with Mark Figgie, MD, and Robert Hotchkiss, MD to develop a new total elbow arthroplasty system. Updated design of the articular geometry and linkage mechanism better distributes load across the joint.
CMC Arthroplasty
Total joint arthroplasty is one solution for severe arthritis of the thumb carpometacarpal (CMC) joint. HSS engineers together with Robert Hotchkiss, MD, Aaron Daluiski, MD, and Daniel Osei, MD, MSc have designed a thumb CMC arthroplasty system. This system has a saddle-on-saddle articulation to replicate native joint function. Leveraging additive manufacturing techniques, the stem of the metacarpal component has variable density for optimized load transfer to the thumb metacarpal bone.
Here is a sample of other designs we’ve been involved with.
- Total Knee Arthroplasty System – multiple HSS surgeons
- Modular Unicompartmental Knee System – Russell Warren, MD and Thomas Wickiewicz, MD
- Pedicle Screw System – Federico Girardi, MD and Andrew Sama, MD
Intellectual Property
Our emphasis on novel, cutting edge research aimed at solving clinical problems in orthopaedics has led to creative innovations that have in turn led to the issuance of United States patents.
9,579,106 Shoulder arthroplasty instrumentation
9,566,022 Apparatus and method for determining 3D load displacement response of a joint
9,545,310 Multi-component non-biodegradable implant, a method of making and a method of implantation
9,408,617 Method for orienting an acetabular cup and instruments for use therewith
9,358,116 Elbow replacement apparatus and methods
9,119,644 Instruments for use in femoroacetabular impingement procedures
9,011,456 Method for orienting an acetabular cup and instruments for use therewith
8,992,625 Posterior stabilized knee prosthesis
8,900,315 Constrained condylar knee device
8,870,964 Prosthetic condylar joints with articulating bearing surfaces having a translating contact point during rotation thereof
8,613,774 Elbow replacement apparatus and methods
8,557,270 Interconnected porous non-degradable poly(vinyl) alcohol implant and method of manufacture
8,440,618 Composition for the attachment of implants to collagen or other components of biological tissue
8,323,281 External fixation devices and methods of use
8,221,317 Expanding cannula and retractor device and methods of use
7,875,081 Posterior stabilized knee prosthesis
7,105,027 Self-aligning knee prosthesis
6,875,234 Dual-radius glenoid prosthetic component for total shoulder arthroplasty
6,592,538 Dynamic orthopedic braces
6,575,980 Method and apparatus for femoral resection
6,311,349 Pelvic positioner
6,159,217 Trochlear clamp
6,154,901 Spinal-surgery table
6,126,692 Retaining mechanism for a modular tibial component of a knee prosthesis
6,010,509 Patella resection drill and prosthesis implantation device
5,997,538 Rotationally ratcheting bone screw
5,916,270 Hip replacement
5,721,334 Process for producing ultra-high molecular weight low modulus polyethylene shaped articles via controlled pressure and temperature and compositions and articles produced there from
5,716,454 Decontamination of devices and instruments contacted with body tissues
5,702,458 Joint prosthesis
5,686,116 Methods of enhancing repair, healing and augmentation of bone implants
5,509,968 Decontamination of orthopaedic implants
5,108,401 Patella cutting clamp
5,015,248 Bone fracture fixation device
4,834,758 Bone prosthesis for the leg and thigh
4,822,364 Elbow joint prosthesis
4,778,475 Femoral prosthesis for total hip replacement
4,731,087 Metatarsal-phalangeal prosthesis
4,714,476 Wrist joint prosthesis
4,408,359 Hip joint prosthesis
4,298,992 Posteriorly stabilized total knee joint prosthesis
4,213,209 Knee joint prosthesis
3,837,009 Knee prosthesis
3,774,244 Knee-joint prosthesis
