A Case of Eosinophilic Granulomatosis with Polyangiitis in a 17-Year-Old Boy
From Grand Rounds from HSS: Management of Complex Cases | Volume 12, Issue 3
Case Report
A 17-year-old boy presented with 1 year of progressive refractory asthma and allergic rhinitis, as well as new onset abdominal pain, nausea, emesis, and diarrhea. Initial workup revealed peripheral eosinophilia, significantly elevated inflammatory markers (maximum erythrocyte sedimentation rate, 113 mm/hr; C-reactive protein level, 234.6 mg/L), and a Blastocystis infection. After completing treatment, he continued to have gastrointestinal (GI) symptoms. He then developed lower extremity sensory and motor deficits consistent with mononeuritis multiplex, a petechial rash, and worsening eosinophilia. An endoscopy and colonoscopy revealed tissue eosinophilia, without evidence of celiac disease, inflammatory bowel disease, or parasitosis. He was found to be positive for MPO (114 AU/mL) and c-antineutrophil cytoplasmic antibody (ANCA) (1:160) and diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA).
After being treated with 3 doses of pulse steroids (1 course of methylprednisolone at 1 g/day for 3 days and 2 courses at 250 mg every 6 hours for 3 days) with improvement, the patient’s abdominal pain and petechial rash recurred. Capsule endoscopy revealed ulcerations, erosions, and pus throughout the small bowel (Fig. 1), consistent with EGPA vasculitis involving the GI tract [1].
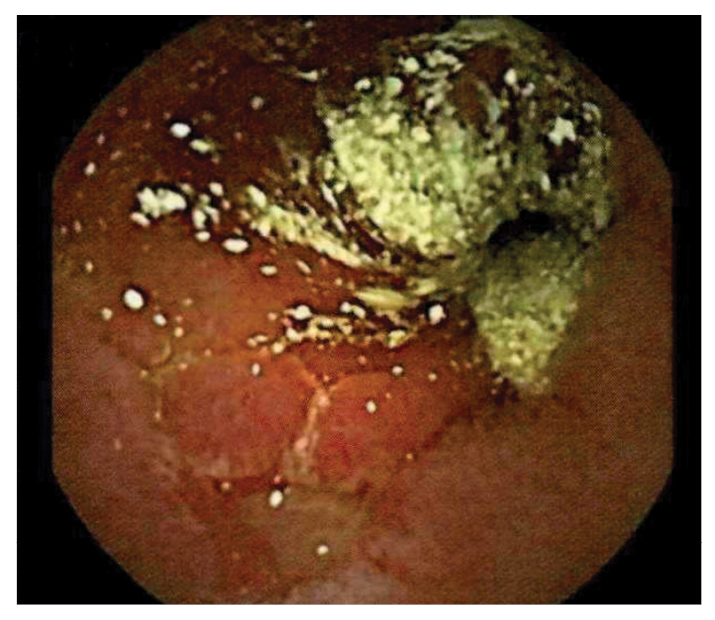
Figure 1: Capsule endoscopy showing frank pus, ulceration, and erythema of the small bowel.
Cyclophosphamide was initiated at 15 mg/kg every 3 weeks for 3 doses. He developed significant and acute testicular pain and a hypertensive emergency (slurred speech and nystagmus). Testicular ultrasound revealed infarction without evidence of torsion, and a computed tomography (CT) scan revealed a hyperdense lesion spanning C4 to C5–6 (Fig. 2), identified on magnetic resonance imaging (MRI) as subarachnoid hemorrhage. The hemorrhage was thought to result from the hypertensive crisis, anticoagulation prophylaxis, or both. Mepolizumab (300 mg SQ monthly) and rituximab (1 g IV twice at 2-week intervals) were added to his induction regimen.
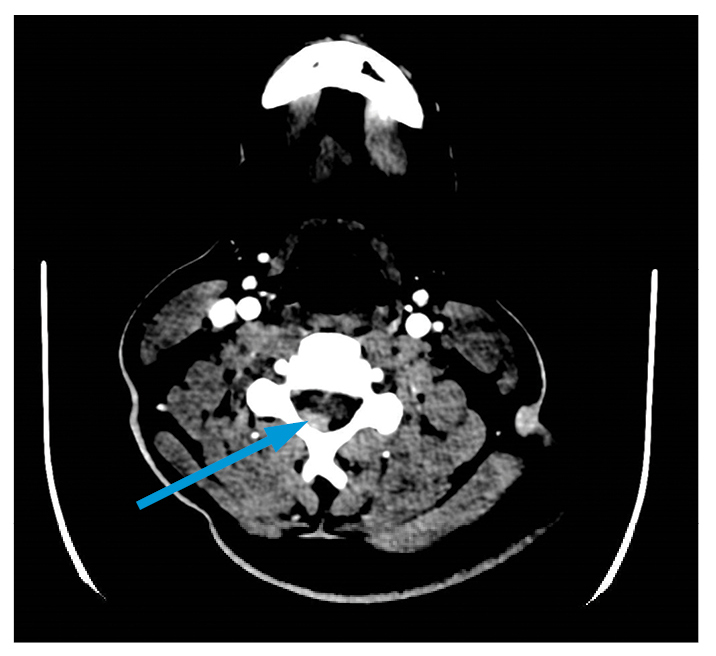
Figure 2: CT angiography of cervical spine with arrow indicating hyperdense focus of right posterior epidural region.
After this treatment escalation, the patient’s eosinophilia, leukocytosis, and inflammatory markers normalized, GI symptoms improved significantly, and petechial rash resolved. At the most recent follow-up, he was doing well on monthly mepolizumab and tolerating an oral steroid taper.
Discussion
ANCA-associated vasculitis (AAV) rarely occurs in childhood, with EGPA being the least common type [2]. Pediatric AAV is more common in girls, with a median age at diagnosis of 10 to 14 years.
EGPA often has a prodrome of atopic respiratory symptoms (e.g., asthma, allergic rhinitis, and nasal polyposis) [3]. As the disease progresses, peripheral eosinophilia develops into an eosinophil rich systemic vasculitis. ANCA is positive in roughly 30% of patients with EGPA [4-5], and patients with positive ANCA appear more likely to develop renal disease [3]. Compared with adults, children with EGPA are more likely to have pulmonary infiltrates and cardiomyopathy and less likely to develop mononeuritis multiplex or myalgia [4]. In one systematic review, 40% of children with EGPA had GI tract involvement [5]. Hypertension may result from cardiac or renal manifestations, but hypertensive emergency is not routinely reported [4].
Early treatment of childhood EGPA is critical, but diagnosis is often delayed [2]. Treatment guidelines for children are extrapolated from studies of adults and patients with other forms of AAV. The mainstay of induction treatment includes glucocorticoids and cyclophosphamide for severe disease. Rituximab may also be used for disease induction and maintenance [6]. Mepolizumab, an anti-interleukin-5 antibody, has also shown reductions in corticosteroid dosing and more weeks in remission, but 65% of one small pediatric EGPA cohort experienced relapse [4]. Children with AAV have more relapses than adults, leading to longer maintenance therapy, more disease sequelae, and higher mortality rates [2,5].
Authors
Megan A. Crippen, MD
Pediatric Rheumatology Fellow
Hospital for Special Surgery
Caitlin A. Ensor, MD, MS
Pediatric Rheumatology Fellow
Hospital for Special Surgery
Attending Physician, Hospital for Special Surgery
Assistant Professor of Pediatrics, Weill Cornell Medical College
References
- Kawasaki K, Nakamura S, Esaki M, et al. Gastrointestinal involvement in patients with vasculitis: IgA vasculitis and eosinophilic granulomatosis with polyangiitis. Endosc Int Open. 2019;7(11):E1333-E1343.
- Jariwala M, Laxer RM. Childhood GPA, EGPA, and MPA. Clin Immunol. 2020;211:108325.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11.
- Fina A, Dubus JC, Tran A, et al. Eosinophilic granulomatosis with polyangiitis in children: Data from the French RespiRare® cohort. Pediatr Pulmonol. 2018;53(12):1640-1650.
- Zwerina J, Eger G, Englbrecht M, Manger B, Schett G. Churg-Strauss syndrome in childhood: a systematic literature review and clinical comparison with adult patients. Semin Arthritis Rheum. 2009;39(2):108-115.
- Akiyama M, Kaneko Y, Takeuchi T. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis: A systematic literature review. Autoimmun Rev. 2021;20(2):102737.


