Allograft-Prosthetic Composite Proximal Humerus Reconstruction in Reverse Shoulder Arthroplasty for Massive Bone Loss
From Grand Rounds from HSS: Management of Complex Cases | Volume 10, Issue 1
Case Report
A 38-year-old woman presented to HSS in 2019 with a poorly functioning reverse total shoulder arthroplasty (rTSA) and pain at rest and with activity. After a malunion following a proximal humerus fracture in 2003, she had undergone several surgical procedures, including a valgus-producing osteotomy of the proximal humerus, removal of hardware, anatomic total shoulder arthroplasty (TSA) with an all-polyethylene glenoid, and finally rTSA with a cemented humeral stem in 2016 (Fig. 1). Symptomatic management had failed in the 3 years prior.
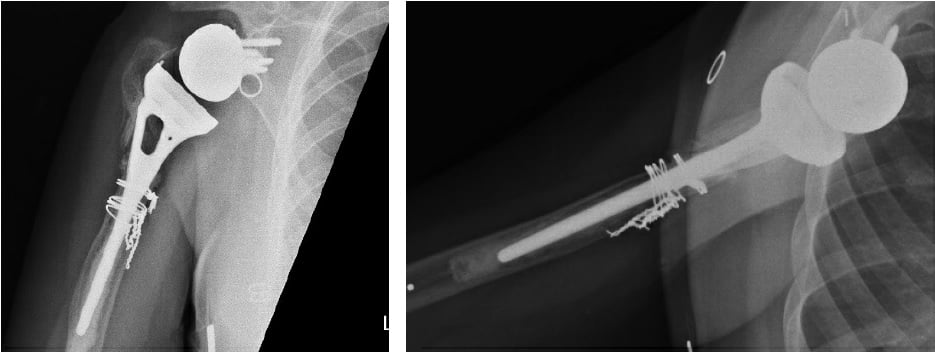
Figure 1: Initial X-rays show rTSA with a well-positioned glenosphere component and proximal humeral bone loss with radiolucency surrounding the humeral stem cement mantle.
Examination revealed numerus extensile healed incisions and visible atrophy of her anterior deltoid, pectoralis major, supraspinatus, and infraspinatus. There was marked tenderness over the bicipital groove and deltoid insertion. Active forward flexion (FF) was limited to 60° and external rotation (ER) to 20°.
Workup for infection showed a mildly elevated (27 mm/hr) erythrocyte sedimentation rate, no leukocytosis, normal C-reactive protein level, and negative aspiration cultures. Magnetic resonance imaging suggested prosthesis loosening, and a computed tomography (CT) scan (Fig. 2) showed significant metadiaphyseal bone stock deficiency.
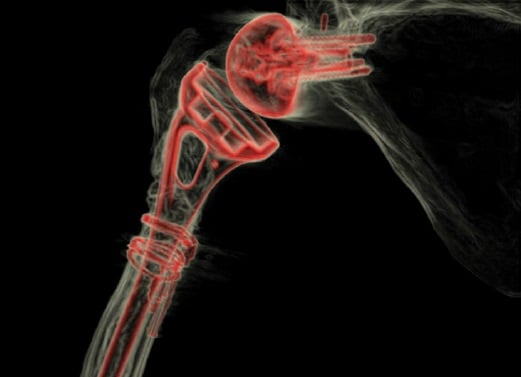
Figure 2: 3-dimensional CT image with post-scan processing to highlight the prosthesis shows a well-fixed glenoid baseplate but near circumferential proximal humeral bone loss at the metadiaphysis.
The patient underwent revision rTSA of the humeral component only, using an allograft-prosthetic composite (APC), rotator cuff repair, and biceps tenodesis. The proximal humerus was exposed via an extended deltopectoral approach, and biopsies and frozen sections were obtained. With no evidence of infection, a single-stage revision proceeded.
The humeral prosthesis was grossly loose proximally but fixed distally, and the surrounding proximal humeral bone was of poor quality and largely nonviable. Metal cerclage wires were removed, as were the prosthesis and 9 cm of proximal humeral bone, with a transverse cut made 1.5 cm distally to the area of bone loss. The stem was extracted with a combination of the Midas Rex drill and narrow flexible osteotomes.
The allograft was then fashioned for use; 9 cm of the proximal humerus was matched to the defect for placement of a revision stem. Of note, the allograft was prepared on the back table to accept the inlay humeral prosthesis and then transferred to the patient to allow for the stem to bypass the allograft–humeral diaphysis interface. The APC was trialed, and satisfied with our length and proximal articulation we fixed an anterior plate both proximally into the allograft and distally into the remaining humeral shaft (Fig. 3).
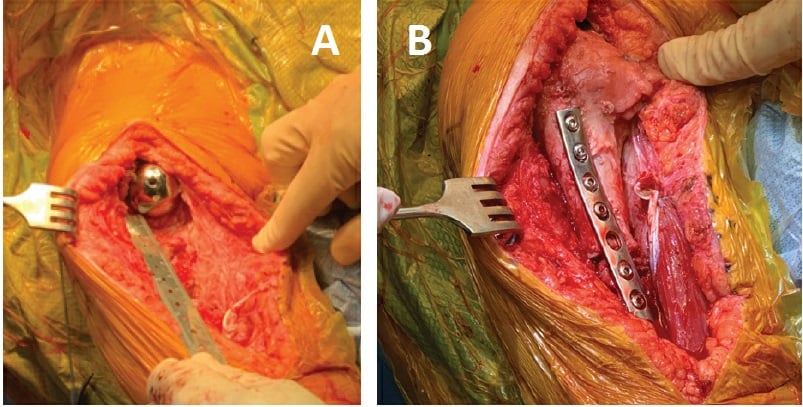
Figure 3: Intraoperative photographs show (a) the humeral defect after component explantation and (b) the final humeral prosthesis with allograft and plate fixation.
Next, the final implant was trialed and a distal screw was removed in order to seat the implant. Satisfied with our length and motion (FF 140°, ER 70°), we cemented the humerus and seated the final components. Final fluoroscopy demonstrated an appropriate intramedullary cement mantle, with some cement extrusion distally out of a breach in the humeral diaphysis incurred during cement removal (Fig. 4). The cement was left in situ to avoid additional morbidity of removal. The posterior rotator cuff tendons (teres minor and infraspinatus) were repaired to the allograft rotator cuff tendon stumps to empower ER.
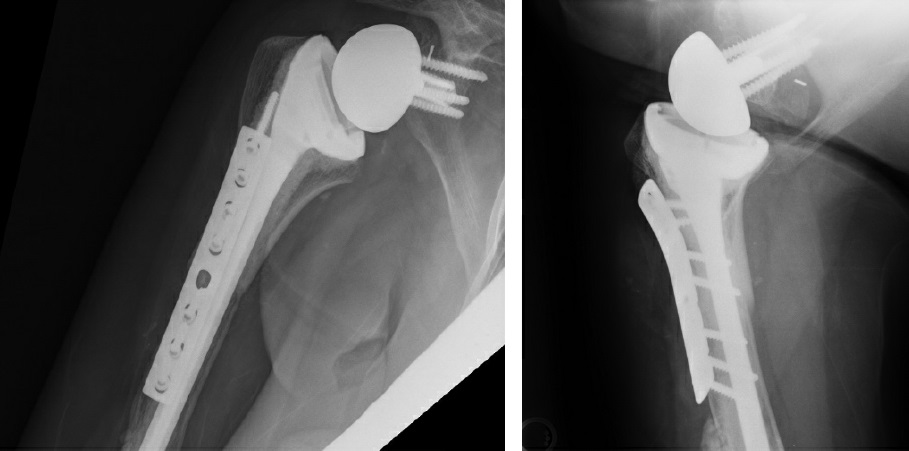
Figure 4: Postoperative X-rays show APC proximal humerus reconstruction.
The patient adhered to our postoperative protocol: weeks 0–6, use sling with distal range of motion (ROM) and Codman pendulum exercises only; weeks 6–12, discontinue sling, with a goal of FF 140° and ER 45°; weeks 12+, continue pursuit of ROM, strength, and endurance. Her postoperative course was uncomplicated aside from feelings of fullness around the area of extramedullary cement extravasation. Radial nerve function was normal. At 1-year follow-up, she reported satisfaction with her outcome, reporting occasional soreness with activity but no resting or nighttime pain. She achieved active forward elevation to 120°, ER to 20°, and internal rotation to the greater trochanter.
Discussion
Failed rTSA has catastrophic implications. Proximal humeral bone loss is an uncommon clinical dilemma, with management options including revision with a tumor prosthesis versus APC. Given our patient’s young age and the probability of future revisions, we opted for APC, a technique adopted from treatments for musculoskeletal tumor resection [1], to preserve as much bone stock as possible. Our case illustrates a pitfall of this operation: cement extravasation from the distal humerus occurring through a breach in the cortex created during cement removal. Fortunately, no substantive deficit occurred as a result. Other potential pitfalls of the procedure include establishing the appropriate length and offset while reapproximating tissue attachments to minimize the risk of dislocation and maximize function [2]. When performed successfully, APC reconstruction is a reasonable option for cases of severe proximal humeral bone loss, showing good functional results with relatively low revision rates and durable results to 10 years [3-7]. A study of upper and lower extremity patients with massive bone loss showed greater durability and lower revision rates with APC than with megaprosthesis reconstruction [8].
References
- Jensen KL, Johnston JO. Proximal humeral reconstruction after excision of a primary sarcoma. Clin Orthop Relat Res. 1995;(311):164-175.
- Sanchez-Sotelo J, Wagner ER, Houdek MT. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. JBJS Essent Surg Tech. 2018;8(1):e3.
- Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415.
- Sanchez-Sotelo J, Wagner ER, Sim FH, Houdek MT. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2017;99(24):2069-2076.
- Reif T, Schoch B, Spiguel A, et al. A retrospective review of revision proximal humeral allograft-prosthetic composite procedures: an analysis of proximal humeral bone stock restoration. J Shoulder Elbow Surg. 2020;29(7):1353-1358.
- Boileau P, Raynier JL, Chelli M, Gonzalez JF, Galvin JW. Reverse shoulder-allograft prosthesis composite, with or without tendon transfer, for the treatment of severe proximal humeral bone loss. J Shoulder Elbow Surg. 2020;29(11):e401-e415.
- El Beaino M, Liu J, Lewis VO, Lin PP. Do early results of proximal humeral allograft-prosthetic composite reconstructions persist at 5-year followup? Clin Orthop Relat Res. 2019;477(4):758-765.
- Gautam D, Arora N, Gupta S, George J, Malhotra R. Megaprosthesis versus allograft prosthesis composite for the management of massive skeletal defects: a meta-analysis of comparative studies. Curr Rev Musculoskelet Med. 2021;14(3):255-270.

