Biomechanics Research
Adult Reconstruction and Joint Replacement
The ultimate goal of the Adult Reconstruction and Joint Replacement group within the biomechanics department is to avoid mechanically related failures of hip and knee arthroplasties, which account for more than 50% of failures. To this end, the group utilizes a combination of computational models, experimental testing, and retrieval analysis to establish the role of patient factors (bone shape and properties, ligament properties, etc.), surgical factors (component choice and positioning, method of fixation, soft tissue management, etc.), and implant design factors (constraint, fixation design, etc.) on the joint kinematics and kinetics, implant fixation, and implant component damage and failure.
Knee Stability
Instability is the fourth most common reason for failure of total knee arthroplasty and the group is focused on understanding the fundamental biomechanical mechanisms for joint stability. The group has developed a novel knee arthrometer that allows measurements of the force required to apply controlled motions to the knee to quantitatively evaluate the knee laxity before and after arthroplasty. With this unique tool, the group aims to understand how objective measures of laxity relate to patient’s perceptions of stability and how implant choice and surgical approach affect joint laxity. In the future, the group expects that such a device will provide surgeons with sufficient information to create patient-specific plans to improve knee stability. To this end, the group is combining the arthrometer research with computational models and cadaveric testing to unveil the contributions of implant design, surgical choices, and patient factors to passive joint stability.
Hip Arthroplasty
Dislocation, where the femoral head dissociates from the acetabular socket, is the second most common reason for failure of total hip arthroplasty. Utilizing a six degree-of-freedom robotic manipulator, the group is reproducing functional positions that lead to dislocation on cadaveric hips to understand how hip soft tissues contribute to resisting dislocation after total hip arthroplasty.
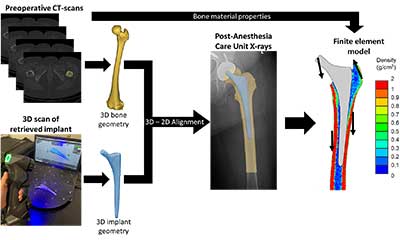
Fracture of the femur around the implant is the fifth most common reason for failure of total hip arthroplasty. The group is utilizing a combination of patient-specific finite element modeling with cadaveric testing to understand how patient factor, implant design, and implant choice and position affect the risk of fracture.

Functional Implant Positioning
Implant loosening is the second most common reason for failure of total knee arthroplasty and the group is focused on determining the optimal position and fixation of implants to maximize longevity. The group is working towards routinely acquiring functional knee information within the clinical pathway of care and using such information to implement patient-specific surgical plans. Some of the active areas of research include radiographic evaluation of the patellofemoral joint in a functional weight-bearing pose, routinely obtain metrics of joint loading like the knee adduction moment by combining biplanar radiographic images with force plate measurements, and routine assessment of bone density in total knee arthroplasty patients through quantitative CT-scans. To leverage these data, the group has developed patient-specific computational models that allow a holistic biomechanical assessment of knee arthroplasties by integrating musculoskeletal models to determine the passive and functional joint mechanics with finite element models to evaluate the bone-implant interaction and implant fixation mechanics.
Retrieval Analysis
The group counts with a well-established retrieval program, established in 1977, through which all joint replacement components removed at Hospital for Special Surgery are collected, cleaned, and cataloged. The group leverages this unique resource to characterize failed implants and obtain insights into the failure modes of joint replacements. This work includes analyzing damage modes of polyethylene components in search for signs of impingement or wear, analyzing bone-contacting surfaces to determine bone ingrowth, or studying fracture surfaces of implants. The group has a particular interest in oxidation of metallic implants, for which they combine retrieval analysis with basic science experiments, where using a corrosion chamber, they can unveil how implant design factors contribute to oxidation.

Engineering Team
Clinical Collaborators
Other Collaborators
Biomaterials for Joint Restoration
The focus of biomaterials research at HSS is on restoring the native mechanics of joints using novel synthetic materials. To achieve this goal a group of engineers, clinicians and scientists have been working on developing novel biomaterial approaches to create synthetic replacements that can immediately function in place of damaged orthopedic soft tissues. The focus of this research is currently on replacing damaged cartilage and meniscus. Cues from development, growth and external forces applied during daily activities are used to drive the development of these functional tissue replacements. Technologies such as 3D printing, microcontrollers and finite element modeling are utilized in the development and generation of these tissue structures.

The ability of these synthetic tissue replacements to restore joint function is screened using an advanced gait simulator and force sensor technology under simulated gait. Bioreactors are also being used to simulate the interaction of cells, native tissue, and the synthetic tissue replacements under the normal loading environment. The outcome of these studies can be used to inform the next generation of tissue replacement designs.
The study of biomaterial design at HSS has led to three patents and a spin-out company focused on replacing cartilage damage.

Engineering Team
Clinical Collaborators
Other Collaborators
Foot & Ankle
The AIM Laboratory for Foot & Ankle Biomechanics is a collaborative group of engineers and clinicians committed to advancing surgical and non-surgical treatments for various pathologies involving the ankle and adjacent joints, including end-stage ankle arthritis, progressive collapsing foot deformity, and hallux valgus. The laboratory research revolves around a robotic gait simulator designed to replicate common patient activities, such as the stance phase of level walking, to study the response of joints during simulated deformities or after different surgical interventions. Other capabilities of the laboratory include joint and plantar pressure measurements using electronic sensors during dynamic activity, digital image correlation of implant and tissue surfaces under load, single-axis and multi-axis mechanical testing, and advanced computational modeling.
One area of research for the laboratory focuses on using a simulated cadaver model of progressive collapsing foot deformity to understand the impact of deformity on joint kinematics and plantar pressures and to study the influence of different surgical interventions. Another research area focuses on recent advances in total ankle arthroplasty devices and the optimization of fixation to improve initial fixation after implantation. Weightbearing computed tomography is also a novel technology that is being used for various projects in the major research areas to help translate results from the laboratory into the clinic to directly improve patient outcomes. The AIM Laboratory for Foot & Ankle Biomechanics has received several grant awards, including recent awards from the NIH, the Feldstein Medical Foundation, and the Weill Cornell Clinical & Translational Science Center.
Engineering Team
Clinical Collaborators
Research in Knee Kinematics and Stability reflects the broader mission of the Biomechanics Department to enable world-class orthopaedic care through innovative engineering.
The knee is a remarkable bioengineering apparatus that enables diverse movements ranging from daily activities like climbing up and down stairs to highly demanding ones like evading a defender while playing soccer or basketball. Performing these activities successfully relies on a complex interplay of the bones, ligaments, and muscles to allow stable motion while tremendous loads cross the knee exceeding seven times body weight in the most strenuous settings.
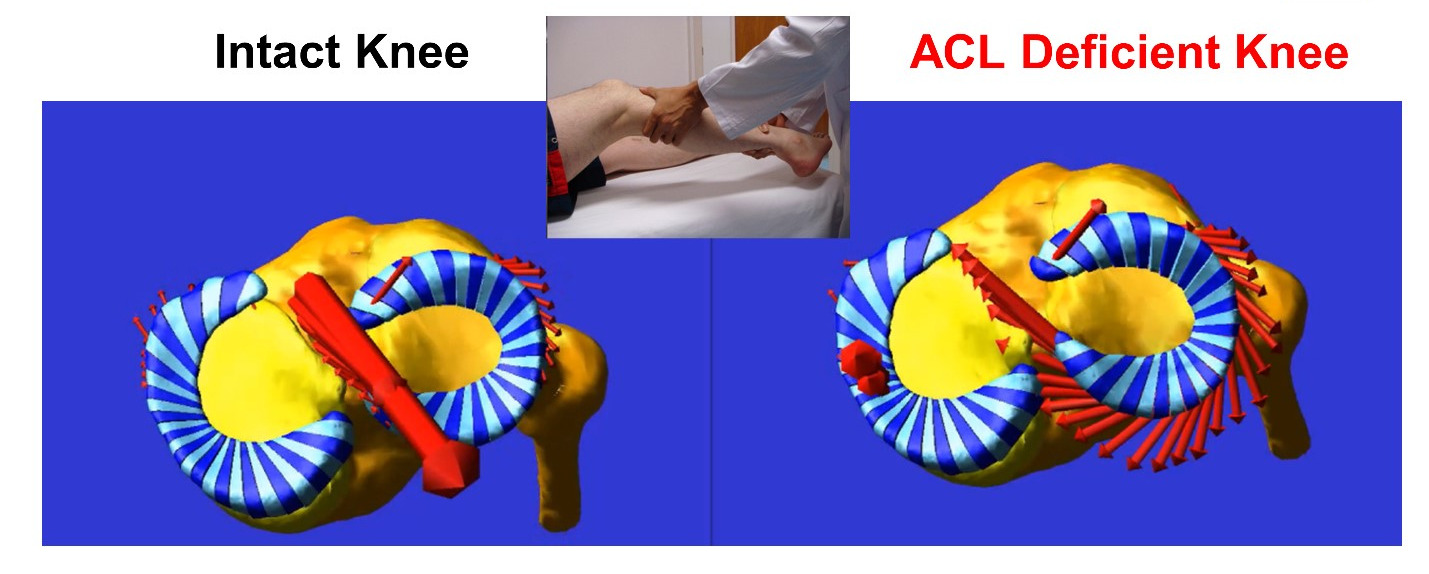
The complex anatomy of the knee and its anatomical heterogeneity across individuals causes knee function to be highly variable from person-to-person. This diversity makes the knee very difficult to treat after injury. Even subtle interpersonal variations in knee anatomy including the shapes of the articulating surfaces and the inherent looseness or tightness of the ligaments (i.e., laxity) can predispose an individual to increased risk of injury like rupture of the ACL or may cause a patient to be dissatisfied following total knee arthroplasty (TKA). Overall, this anatomical diversity may contribute to the high variability in outcomes seen in current therapies and surgeries and can leave such one-size-fits all treatments doomed to fail in some individuals.
Our goal is to understand how anatomical nuances contribute to knee motion and to loading of key support structures of the knee like the anterior cruciate ligament (ACL). To date, the mechanistic link between knee anatomy and associated mechanics including ACL force and knee movements (stability) is not well understood, especially in high-risk populations like young, active females playing sports like soccer and basketball. Our team’s efforts are founded in the belief that understanding this mechanistic link will help guide the design of more effective personalized screening programs, injury prevention protocols, and surgical treatments, which target the specific mechanisms of action that predispose an individual to injury or to a poor outcome after surgery.
To develop this basic understanding, we study knee biomechanics in the context of health, injury, disease, replacement, and reconstruction using a diverse array of model systems including in vitro (cadaver), in vivo (clinical), and in silico (computer) approaches.
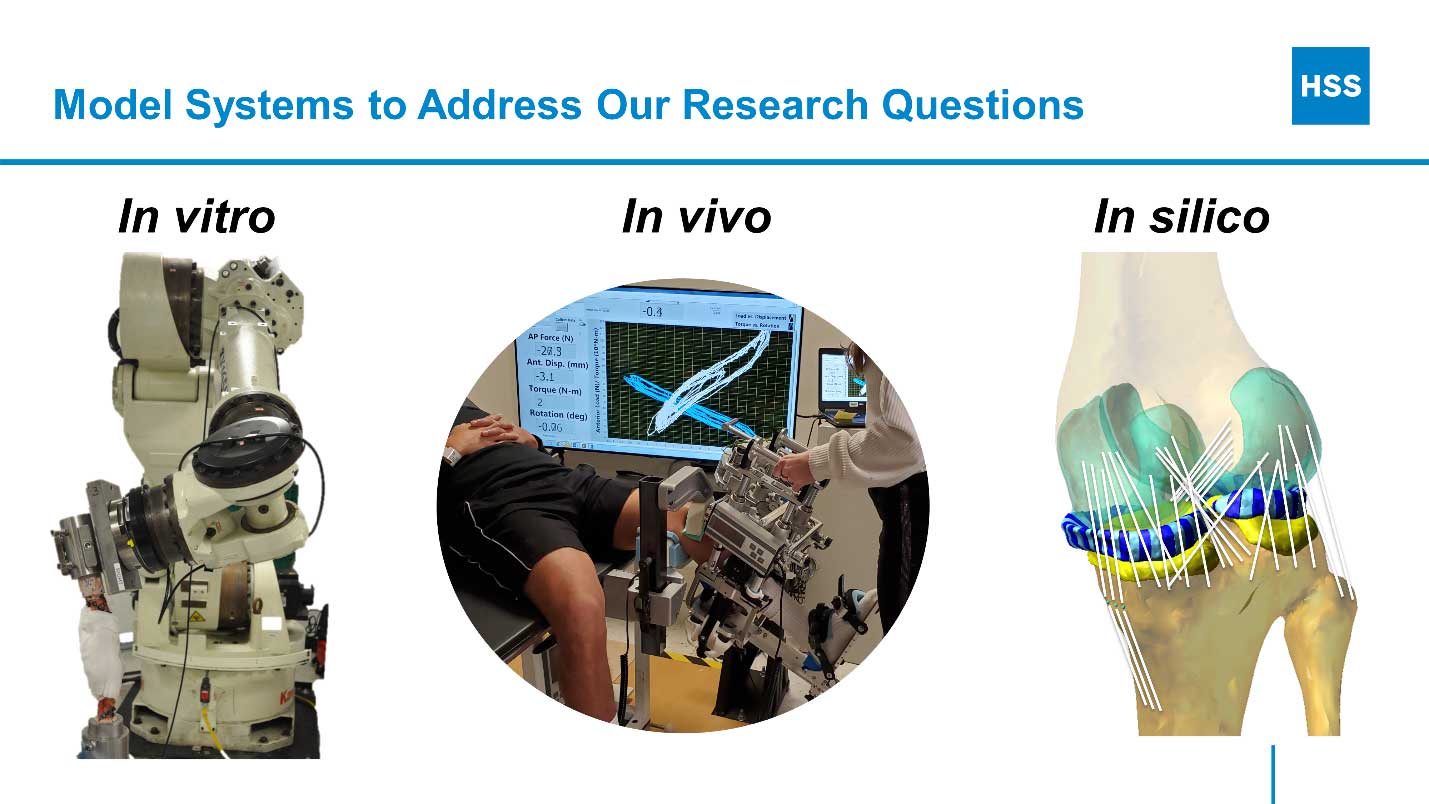
Collaboration with clinical colleagues in orthopedic surgery and rehabilitation and partnership with experts in medical imaging, epidemiology, and statistical modeling are existential to our efforts. This integrated approach capitalizes on the strengths of each method and team member such that the whole is greater than the sum of its parts.

ACL Biomechanics Research Team Leadership
Strong connections with The School of Engineering at Cornell University via the NIH-funded HSS-Cornell Center for Advanced Materials and Engineering in Orthopaedics (CAMEO) and through the Meinig School of Biomedical Engineering provide engineering support for our efforts and experiential learning opportunities in musculoskeletal medicine for Master’s students via focused design projects.

Translation of our work to clinical practice is an essential part of ‘closing the research loop’. We aim to develop scalable, transferable, widely used technologies to positively impact patient care. Therefore, beyond basic knowledge, we utilize preclinical data to drive clinical trials as a prerequisite for translation to clinical practice. Such trials will use in silico estimates of knee mechanics as the primary outcome to compare standard protocols and treatments for ACL rupture or of TKA implantation with novel mechanics-based approaches. Specific goals are to: 1) enhance ACL injury prevention protocols; 2) design more sensitive and specific ACL injury screening tools; 3) develop novel surgical treatments customized to an individual's sex, sport, age, level of play, and injury status; and 4) devise novel implant designs and surgical techniques to reduce incidence rates of knee instability following total knee replacement.
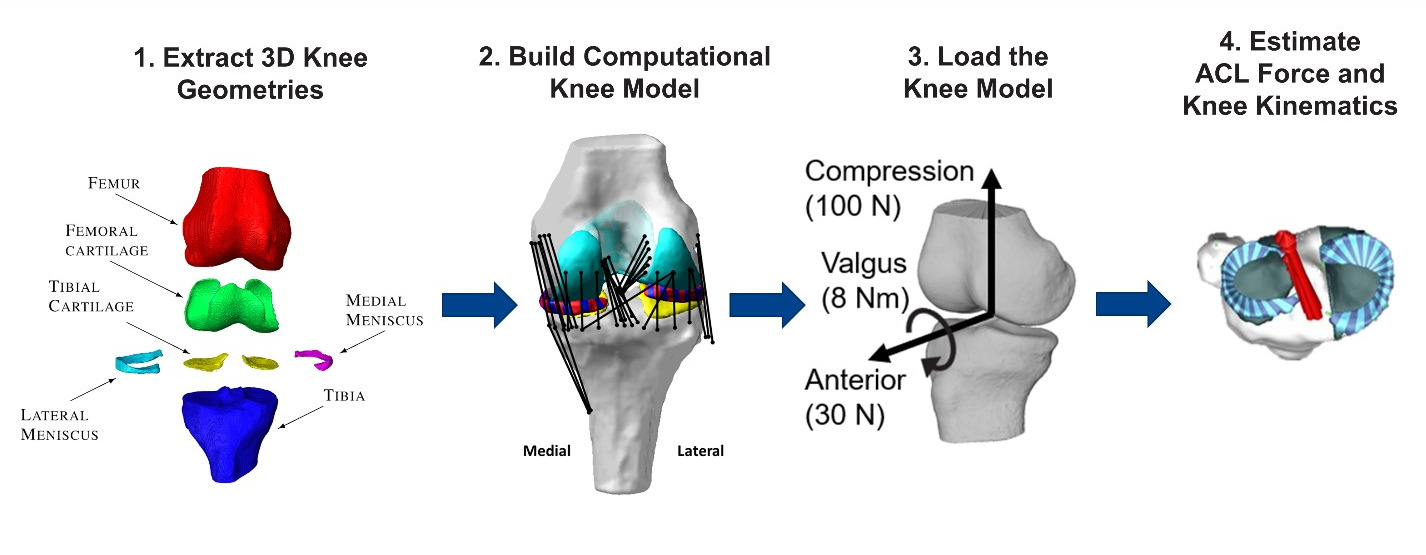
On-going projects include the following: 1) Identifying more sensitive and specific biomechanical risk factors for ACL injury via population-based computational multibody dynamics modeling of the knee. 2) Devising return-to-play criteria following ACL reconstruction surgery via integrated 3D MRI-imaging of graft structure and composition, in silico prediction of ACL graft forces during high demand activities, and objective assessment of 3D knee stability via in vivo robotic testing. 3) Linking patient-based measured of knee laxity and computational estimates of knee stability and tissue loading to patient satisfaction post-TKA to derive optimal surgical treatment plans.
We believe that physics-based computational models implemented in the clinic will provide a powerful way to virtually ‘rehearse’ surgical scenarios prior to intervening to inform the surgeon of the most appropriate procedure to reduce likelihood of reinjury. Accordingly, this approach will help ‘level the playing field’ and reduce disparities in injury prevention and treatment of young women who suffer ACL rupture and increase satisfaction after TKA. Finally, the use of high throughput computational modeling and simulation will enable in silico clinical trials whereby estimates from computational models (e.g., ACL force) are used as biomarkers for safety and efficacy of treatments while reducing or replacing human participants.
Another research focus is improving reproducibility of research findings in modeling and simulation (M&S) of the knee joint through a multi-institutional and open collaboration among experts in the field. In particular, the subjective decisions of the model developer, the 'art' of M&S, may be a critical barrier to achieving reproducibility impeding adoption of these powerful tools. This collaboration addresses this issue through prospective documentation of modeling workflows, head-to-head comparison of model predictions across groups, and widespread dissemination of model results.
We present our research at national and international scientific meetings spanning technical and clinical applications and orthopedic science. Examples of conferences where we have presented our work are: the Annual Meeting of the Orthopaedic Research Society-the premier scientific meeting in orthopaedics; the biennial meeting of the ACL Study Group- a meeting of international leaders in ACL reconstruction; ISAKOS - a meeting dedicated to the science and practice of knee surgery and orthopaedic sports medicine; The World Congress of Biomechanics- a meeting of the world biomechanics community that is held once every four years; and the biennial Computer Methods In Biomechanics and Bioengineering Conference- an international technical conference focusing on modeling and simulation.

We publish our work in leading peer-reviewed scientific, technical, and clinical journals.
Shoulder
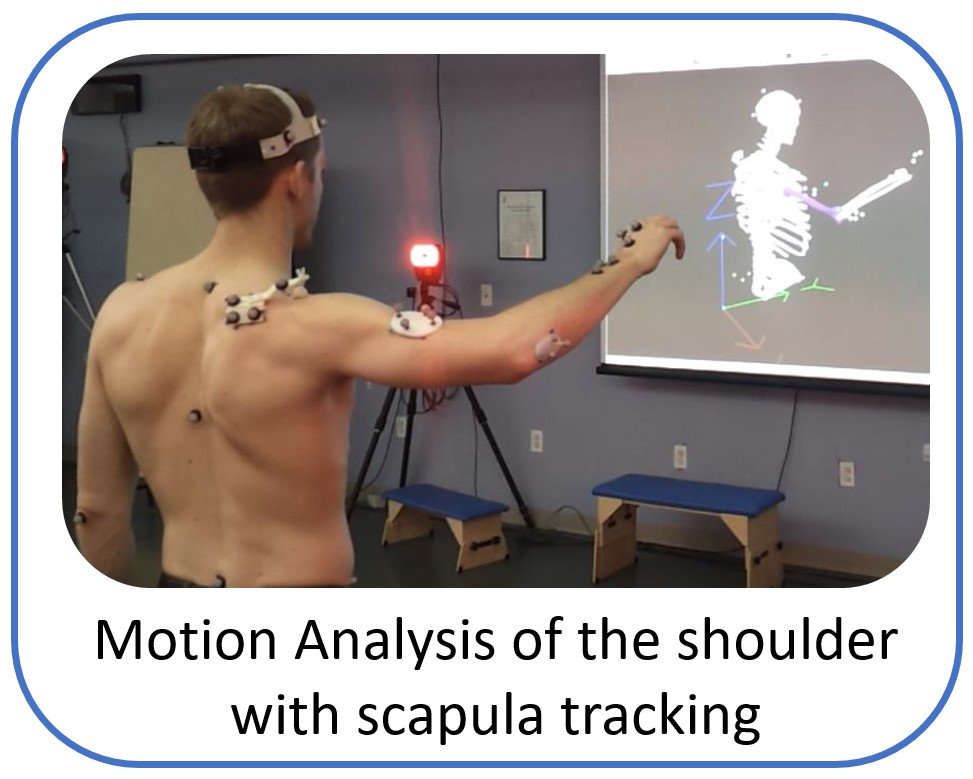
The research work and ideas of the Shoulder Biomechanics group aim to develop world-class products and services that can improve quality of life for patients with painful musculoskeletal disorders in upper extremity. The group uses state of the art research tools like advanced musculoskeletal modelling, motion analysis with electromyography and cadaveric testing, to explore and answer challenging clinical questions and create application that assist the physicians and physiotherapists to optimize clinical outcome and rehabilitation.
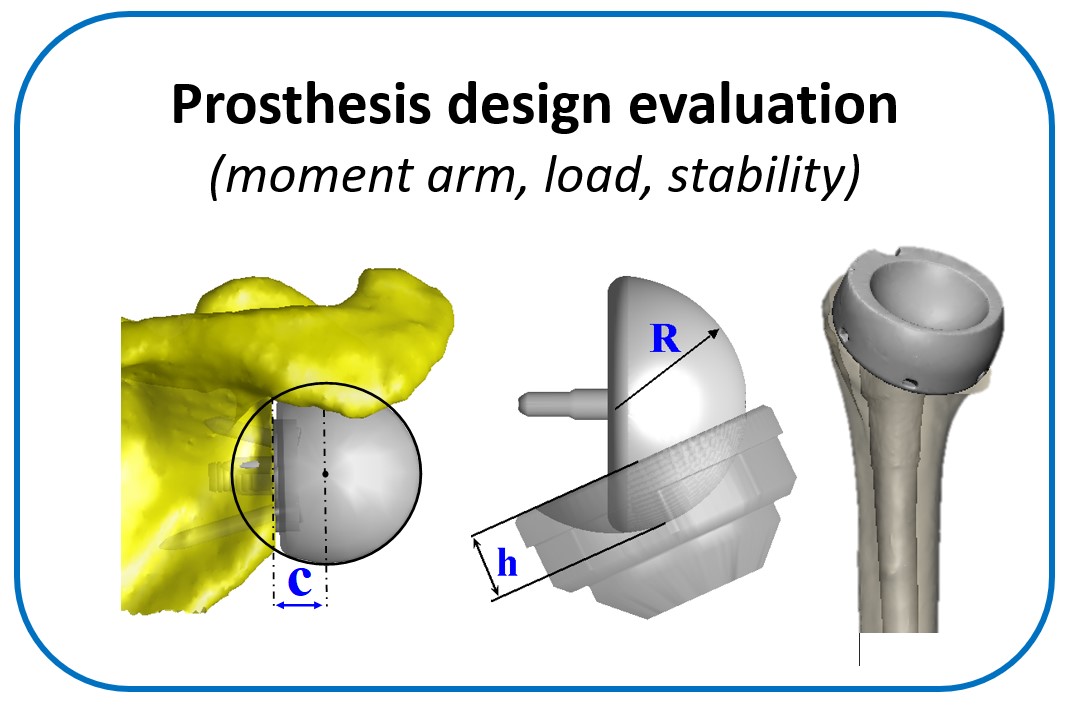
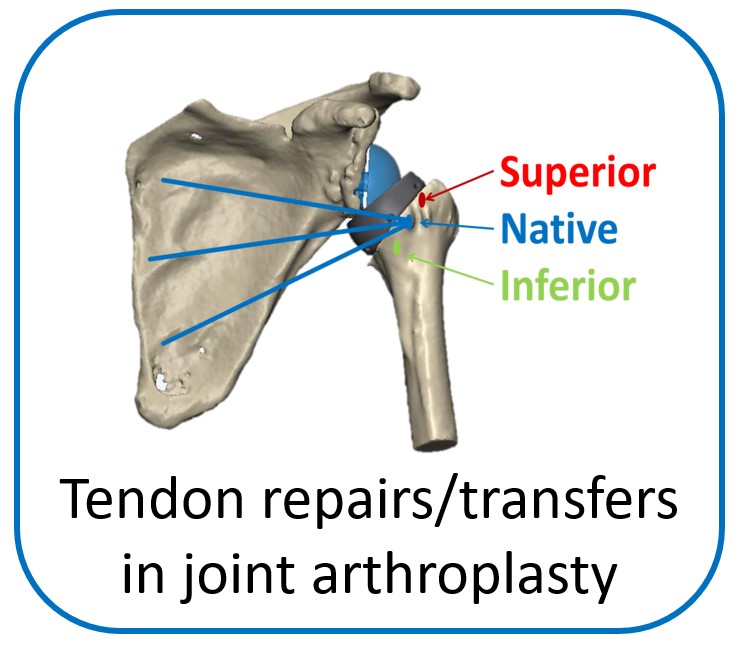
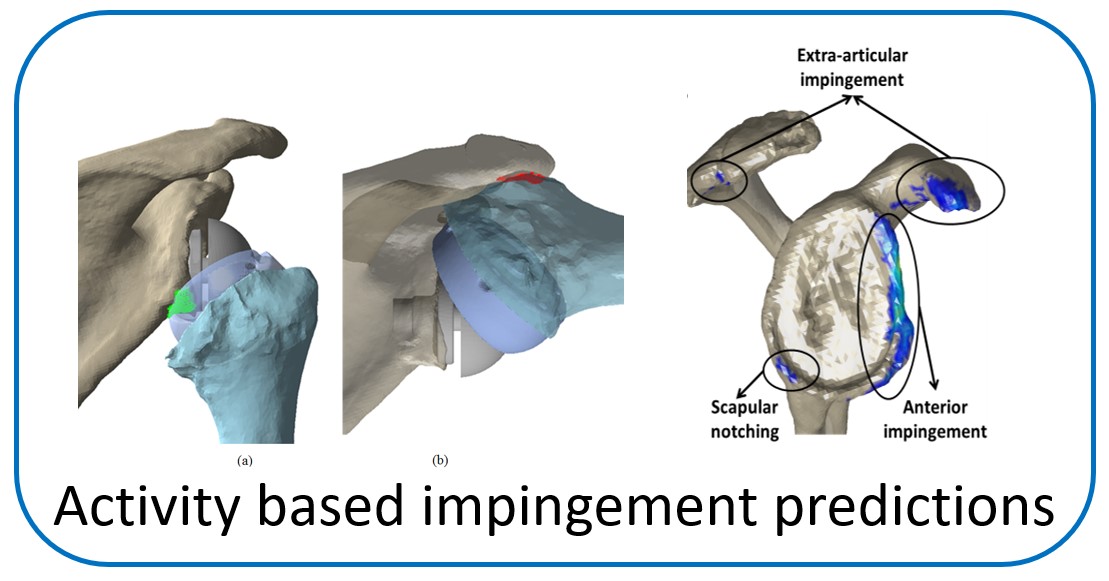
One of the main objectives of the group is to develop and validate novel pre-operative planning tools that will help shoulder surgeons optimize post-operative function after Reverse or Total Shoulder Arthroplasty in osteoarthritic shoulders. The group has developed a framework that analyzes the performance of several biomechanical factors of the shoulder joint replacement (e.g. muscle and joint forces, impingement, muscle moment arm) and to provide the surgeons with prediction about post-operative range of motion based on surgical implantation, prosthetic design and how tendon transfers can enhance post-operative function in patients with limited pre-operative motion.
The group is also working to understand how structure and function in non-arthritic shoulders is compromised after common traumatic sports injuries (like anterior instability after dislocation). The group uses similar cadaveric and modelling research tools to evaluate how common clinical practices restore joint stability and how patients can return to normal activities or sport.