Information for HSS Patients about the Exactech Recall
On February 8, 2022, Exactech, Inc., an orthopedic device company headquartered in Gainesville, Florida, USA, issued a recall of implant components used in several of its knee and ankle systems, related to Exactech’s packaging process.
Many hospitals and surgeons around the world used Exactech knee and ankle implants for replacement surgeries, including Hospital for Special Surgery (HSS).
The recall was expanded on July 22, 2022, to include some Exactech hip implants; on March 6, 2024, to include some shoulder implants; on April 18, 2024, to include some patella implants; and on December 31, 2024, to include additional hip implants. The expansions of the recall were related to the same packaging issue as the knee and ankle components.
Who is affected by this recall?
Most HSS patients who received implants are not affected by the Exactech recalls. The recalls affect fewer than one in five patients who received knee, hip, shoulder or ankle implants at HSS between 2004 and 2021. Among patients who are affected, the failure rate of these implants is very low. HSS is committed to helping affected patients understand the symptoms that may indicate the need for medical assessment.
HSS has contacted all affected patients
HSS sent notifications to its patients impacted by the Exactech knee, ankle, shoulder and hip implant recalls.
Explanation of the Exactech Recall
Knee Implant
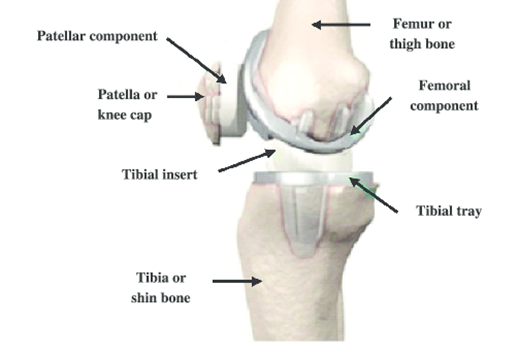
As shown in the diagram below, a standard knee replacement has four parts:
- Femoral component — this is the metal piece that attaches to the thigh bone, also known as the femur.
- Tibial tray — this is the metal piece that fits into the shin bone, also known as the tibia.
- Patellar component — this is the piece of plastic that fits onto the kneecap, also known as the patella.
- Tibial insert — this is a plastic insert made out of polyethylene that fits between the femoral component and tibial tray and acts as the new cushion or cartilage for the replaced knee joint.
During a recent review of its knee implant manufacturing process, Exactech learned that one of the packaging layers for the tibial insert has been out of specification and may allow oxygen from the air to diffuse into this tibial insert prior to it being implanted in a patient's knee. If a large amount of oxygen diffuses into the tibial insert while it's being stored and before it is implanted, this can lead to a process called oxidation. Oxidation can cause the tibial insert to wear out earlier than expected or to become damaged after it is implanted into the patient's body.
Ankle Implant
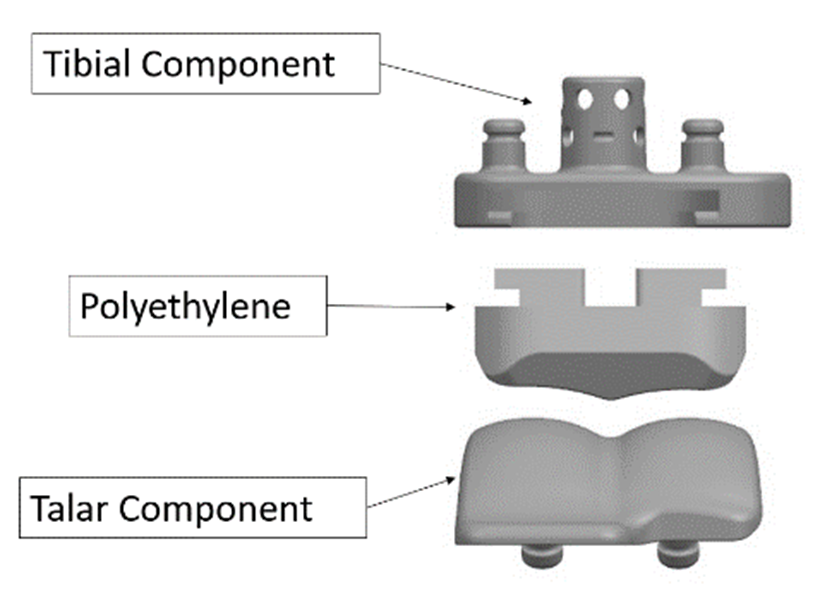
As shown in the diagram below, a standard ankle replacement has three parts:
- Tibial component — this is the metal piece that attaches to the shin bone, also known as the tibia
- Talar component — this is the metal piece that fits into the foot bone, also known as the talus
- Polyethylene (plastic) insert — this is the plastic insert that fits between the tibial component and the talar component and acts as the new cushion or cartilage for the replaced ankle joint
During a recent review of its ankle implant manufacturing process, Exactech learned that one of the packaging layers for the plastic insert has been out of specification and may allow oxygen from the air to diffuse into this plastic insert prior to it being implanted in a patient's ankle. If a large amount of oxygen diffuses into the plastic insert while it's being stored and before it is implanted, this can lead to a process called oxidation. Oxidation can weaken the plastic insert and cause it to wear out earlier than expected or cause it to become damaged after it is implanted into the patient's body.
Hip Implant
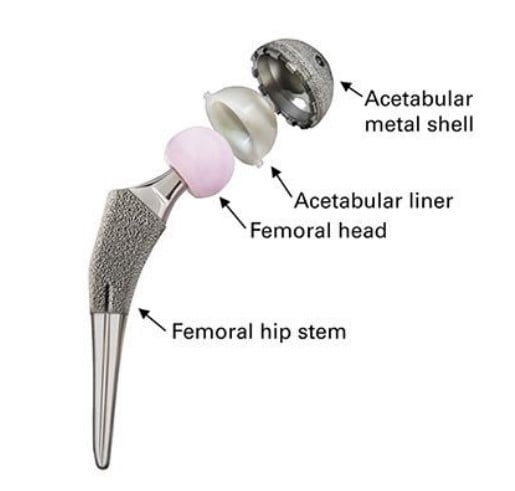
As shown in the diagram below, most standard hip replacements contain four critical parts:
- Acetabular metal shell - this is the metal “socket” that goes into your native hip socket.
- Acetabular liner (plastic / polyethylene) liner - this is the new “cushion” that replaces the damaged cartilage.
- Femoral head - this is the new ball of your hip joint, usually made of ceramic or metal.
- Femoral hip stem - this is the component that fits into your thigh bone and secures the new ball.
During a recent review of its hip implant manufacturing process, Exactech learned of an issue with the packaging of the plastic insert which can allow oxygen from the air into the plastic insert prior to it being implanted. If a large amount of oxygen diffuses into the plastic insert while it is being stored and before it is implanted, this can lead to a process called oxidation, which can cause the plastic to wear out earlier than expected or to become damaged after it is implanted into the patient’s body.
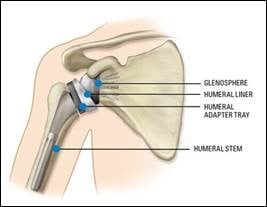
Shoulder Implant
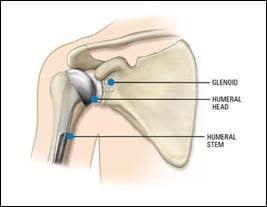
As shown in the diagram below, a standard shoulder replacement has four parts:
- Glenosphere – this is the metal ball that attaches to the shoulder socket, also known as the glenoid.
- Humeral liner – this is the plastic piece that acts as the new socket, fitting into the humeral adapter tray to allow smooth movement.
- Humeral adapter tray - this is the metal piece that connects the humeral liner to the humeral stem.
- Humeral stem - this is the metal piece that fits into the upper arm bone, also known as the humerus.
TSR System
Reverse TSR System
During a recent review of its shoulder implant manufacturing process, Exactech learned that one of the packaging layers for some Equinoxe Shoulder System glenoid components and humeral liners were packaged in non-conforming bags that were missing one of the oxygen barrier layers that protect the devices from oxidation. Non-conforming bags may allow oxygen from the air to contact the plastic (polyethylene) component of the Equinoxe Shoulder System before it is implanted into a person's body.
Unlike the similar non-conforming packaging of Exactech polyethylene knee, ankle and hip components, the shoulder is biomechanically different than the hip, knee or ankle. Less body weight is placed on the shoulder with the types of activities that involve use of the shoulder than the hips, knees or ankles, therefore there is less stress on a shoulder implant. Exactech reviewed joint revision data in the US and in Europe, as well as peer-reviewed literature, and found revisions for polyethylene wear, dissociation, osteolysis, and fracture are rare with shoulder arthroplasty across all manufacturers. Additionally, Exactech performed oxidation and strength tests on the shoulder implants packaged in non-conforming packaging; they found low oxidation index unlikely to have a substantially negative impact on mechanical behavior.
What should you do?
The symptoms of premature wear include unusual pain or aching, swelling, redness, stiffness, difficulty moving and/or instability.
If you do not have any of these symptoms, you can just follow-up at your regularly scheduled time interval. We expect the percentage of patients to develop any problems due to the issues identified in this recall to be very low.
If you are experiencing any of these symptoms, please contact your surgeon’s office at HSS.
Exactech Bankruptcy
On October 29, 2024, Exactech filed for Chapter 11 Bankruptcy.
For those patients who registered with Broadspire for reimbursement of out-of-pocket expenses related to the recall, the program was discontinued with the Exactech bankruptcy.
Exactech identified Kroll to assist in the administration of claims submissions to the Bankruptcy Court. Instructions for submitting claims can be found on Exactech's restructuring website: https://restructuring.ra.kroll.com/Exactech. Any questions can be directed to the restructuring hotline 833.918.0986 (US/Canada Toll-Free) and +1.646.781.8728 (International).
Additional information about these services and frequently asked questions can be found on the Exactech website at: www.exac.com/recall.
Contact Information
![]()
HSS Patient Access
We understand you may have questions and concerns. We are here to assist.
If you are seeking assistance related to the recalls, please contact your surgeon’s office at HSS or “HSS Connect” at 212.606.1555. HSS Connect can help you identify your surgeon or, if your surgeon is no longer at HSS, which surgeon you should contact.
