
Chitra Dahia, PhD
About Dr. Dahia
Research
Spine Development and Regeneration Lab
The main focus of Dahia lab is to understand the role of major cell signaling pathways, particularly Shh, Wnt, BMP and TGFβ in postnatal development, differentiation, aging and degeneration of the intervertebral disc (IVD), and translate this to develop approaches for regeneration of the IVD and to treat lower back and disc related disorders.
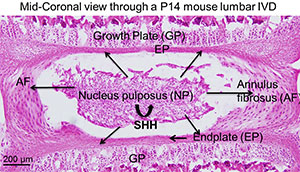 IVDs are cartilagenous tissue present between each vertebra and its growth plates (GP). Each IVD consists of a central semi-liquid nucleus pulposus (NP), surrounded by a multi-layered annulus fibrosus (AF), and connected to the bodies of the adjacent vertebral bodies by cartilagenous end plates (EP). Together, these components form a strong joint that resists both tension and compression forces between vertebrae. Degenerative disc disease (DDD) is a major cause of lower back pain, and other neurological symptoms leading to a decreased quality of life. DDD is extremely common, affecting as many as one in seven people. However, how is it triggered is not known. The treatment costs are high, and often include surgical intervention. However, treatment is essentially palliative, since it treats the effects of disc degeneration rather than the causes.
IVDs are cartilagenous tissue present between each vertebra and its growth plates (GP). Each IVD consists of a central semi-liquid nucleus pulposus (NP), surrounded by a multi-layered annulus fibrosus (AF), and connected to the bodies of the adjacent vertebral bodies by cartilagenous end plates (EP). Together, these components form a strong joint that resists both tension and compression forces between vertebrae. Degenerative disc disease (DDD) is a major cause of lower back pain, and other neurological symptoms leading to a decreased quality of life. DDD is extremely common, affecting as many as one in seven people. However, how is it triggered is not known. The treatment costs are high, and often include surgical intervention. However, treatment is essentially palliative, since it treats the effects of disc degeneration rather than the causes.
Research in Dahia lab aims to identify the molecular mechanisms of postnatal growth, differentiation and aging of the IVD using mouse model system, and how these mechanisms are down-regulated, leading to age-related changes in the IVD. We have developed the mouse lumbar IVD as a model, and successfully shown that it can be used to identify the signaling pathways in the IVD that control postnatal growth and differentiation. We have found that during postnatal growth, the NP acts as a signaling center that controls both growth and differentiation of the postnatal IVD. NP cells express SHH, and blockade of Shh signaling both in vitro and in vivo shows that it is essential for cell proliferation and differentiation of the IVD. NP cells also express several WNT ligands, which are required to maintain Shh signaling in the IVD. Both these signaling pathways, and their downstream targets are down-regulated by the end of the growth period. Our studies show that Shh signaling, and the expression of differentiation markers, can be re-activated after the growth period by activation of canonical Wnt signaling. This suggests the potential of Shh and Wnt signaling pathways in regeneration of IVD.
The long-term goal is to identify potential biological approaches to therapy, using the same pathways that control IVD growth and differentiation during the early postnatal stages.
Candidates interested in PostDoctoral positions may email a detailed resume, electronic reprints of recent publications, and names and contact information for three references to dahiac@hss.edu
Videos
Credentials
Appointments
Associate Scientist, HSS Research Institute, Hospital for Special Surgery
Associate Professor of Cell and Developmental Biology, Cell and Developmental Biology ,Weill Cornell Medical College
Associate Professor, BCMB Program, Weill Cornell Medicine, Graduate School of Medical Sciences
Languages
English
Publications by Dr. Dahia
Selected Books/Chapters
Chitra Lekha and A. J. Rao. Development of male contraceptives: approaches, associated problems and possible solutions; in “Hormone Biotechnology,” pp 3-16 2007, published by Daya Publishing House New Delhi, Ed. S.K.Maitra.
Selected Publications
Mohanty S, Pinelli R, Pricop P, Albert TJ, Dahia CL. (2019) Chondrocyte-like nested cells in the aged intervertebral disc are late-stage nucleus pulposus cells. Aging Cell 2019. Jul 10:e13006. DOI: 10.1111/acel.13006. [Epub ahead of print]. PMID: 31290579
Mohanty S, Pinelli R, Dahia CL. (2019) Characterization of Krt19CreERT allele for targeting the nucleus pulposus cells in the postnatal mouse intervertebral disc. J Cell Physiol. 2019 Jun 11. doi: 10.1002/jcp.28952. [Epub ahead of print]. PMID: 31187500
Mohanty S, Dahia CL. (2019) Defects in Intervertebral Disc and Spine During Development, Degeneration, and Pain: New Research Directions for Disc Regeneration and Therapy. Wiley Interdiscip Rev Dev Biol. 2019 Jul;8(4):e343; DOI: 10.1002/wdev.343. Epub 2019 Apr 11. PMID: 30977275. PMCID: PMC6565447. “Chapter review by invitation”
Vincent K, Mohanty S, Pinelli R, Bonavita R, Pricop P, Albert TJ, Dahia CL. (2019) Aging Of Mouse Intervertebral Disc And Association With Back Pain. Bone. 2019 Jun;123:246-259. doi: 10.1016/j.bone.2019.03.
037. Epub 2019 Mar 29. PMID:30936040. PMCID: PMC6549718.
Rajesh D, Dahia CL. (2018) Role of Sonic Hedgehog Signaling Pathway in Intervertebral Disk Formation and Maintenance. Curr Mol Bio Rep. 2018 Dec; 4(4):173-179. DOI:10.1007/s40610-018-0107-9. Epub 2018 Oct 24. PMID: 30687592. PMCID: PMC6345536. “By invitation”
Seguin CA, Chan D, Dahia CL, Gazit Z. (2018) Latest advances in intervertebral disc development and progenitor cells. JOR Spine. 2018 Sep;1(3):e1030. DOI:10.1002/jsp2.1030. Epub 2018 Aug 28. PMID: 30687811. PMCID: PMC6338208. “By invitation”
Dahia CL*, Iatridis JC, Risbud MV. (2018) New Horizons In Spine Research: Disc Biology, Tissue Engineering, Biomechanics, Translational And Clinical Research. JOR Spine. 2018 Sep;1(3):e1032. DOI:10.1002/jsp2.1032. Epub 2018 Aug 6. PMID: 30687810. PMCID: PMC6345533. “By invitation”. *corresponding author
Bonavita R, Vincent K, Pinelli R, Dahia CL. (2018) Formation of the sacrum requires down-regulation of sonic hedgehog signaling in the sacral intervertebral discs. Biol Open. 2018 Jul 24;7(7). pii: bio035592. DOI: 10.1242/bio.035592. PubMed PMID: 29784673. PMCID: PMC6078355.
Carbone A, Carballo C, Ma R, Wang H, Deng X, Dahia C, Rodeo S. (2016) Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: Evaluation in a rat ACL reconstruction model. J Orthop Res. 2016 Apr;34(4):641-9. Epub 2015 Nov 25. DOI: 10.1002/jor.23066. PMID: 26447744. PMCID: PMC6345400.
Zheng HF*, Forgetta V*, Hsu YH*, Estrada K*, Rosello-Diez A*, Leo PJ*, Dahia CL*, et al., (2015) Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015 Oct 1;526(7571): 112-7. DOI: 10.1038/nature14878. Epub 2015 Sep 14. PMID: 26367794. PMICD: PMC4755714.
*These authors contributed equally to this work. CLD also contributed as a PI.
Winkler T, Mahoney E, Sinner D, Wylie C, Dahia CL. (2014) Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PLoS One, 2014 Jun 3;9(6): e9844. DOI: 10.1371/journal.pone.0098444. eCollection 2014. PMID: 24892825. PMICD: PMC4043533.
Dahia CL*, Mahoney E, Wylie C*. (2012) Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS ONE. 2012;7(4): e35944. DOI:10.1371/journal.pone.0035944 Epub 2012 Apr 27. PMID: 22558278. PMICD: PMC3338762. * co-corresponding authors
Dahia CL, Mahoney E, Durrani A, Wylie C. (2011) Intercellular signaling pathways active during and after growth and differentiation of lumbar vertebral growth plate. Spine (Phila Pa 1976). 2011 Jun 15;36(14):1071-80. DOI: 10.1097/BRS.0b013e3181f7a3ca. PMID: 21245780
Dahia CL, Sarkar MN, Rao AJ. (2011) A Comparative Analysis of Expression of Selected Genes during Induction of Differentiation in Neonatal Rats by Deprival of FSH or by Hyperthyroidism. The Open Andrology Journal. 2011 (3):14-24. Epub 2011 July 26. DOI: 10.2174/1876827X01103010014
Dahia CL, Mahoney E, Durrani A, Wylie C. (2009) Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine (Phila Pa 1976). 2009 Mar 1;34(5):447-55. DOI: 10.1097/BRS.0b013e3181990c64. PMID: 19247165.
Dahia CL, Mahoney E, Durrani A, Wylie C. (2009) Intercellular signaling pathways active during intervertebral disc growth, differentiation and aging. Spine (Phila Pa 1976). 2009 Mar 1;34(5):456-62. DOI: 10.1097/BRS.0b013e3181913e98. PMID: 19212276.
Dahia CL, Rao AJ. (2009) Identification of Intracisternal A-Particle-Like Element as an FSH- regulated Transcript in Immature Rat Sertoli Cells. The Open Andrology Journal. 2009 (1):10-19. Epub 2009 Jun 9. DOI: 10.2174/1876827X0090101001
Dahia CL, Petruz P, Hall SH, Rao AJ. (2008) Effect of deprivation of endogenous follicle stimulating hormone on rat epididymis: a histological evaluation. Reproductive BioMedicine Online. 2008 Sep;17(3) 331-7. PMID: 18765003.
Dahia CL, Rao AJ. (2006) Demonstration of follicle-stimulating hormone receptor in cauda epididymis of rat. Biology of Reproduction. 2006 Jul;75(1):98-106. DOI: 10.1095/biolreprod.105.047704. Epub 2006 Apr 5. PMID: 16598027.
Dahia CL, Rao AJ. (2006) Regulation of FSH receptor, PKIbeta, IL-6 and calcium mobilization: Possible mediators of differential action of FSH. Molecular and Cellular Endocrinology. 2006 Mar 9;247(1-2):73-81. DOI: 10.1016/j.mce.2005.10.029. Epub 2006 Jan 6. PMID: 16406266.
For more publications, please see the PubMed listing.
Industry Relationships
Industry Relationships
One of the goals of HSS is to advance the science of orthopedic surgery, rheumatology, and related disciplines for the benefit of patients. Research staff at HSS may collaborate with outside companies for education, research and medical advances. HSS supports this collaboration in order to foster medical breakthroughs; however, HSS also believes that these collaborations must be disclosed.
As part of the disclosure process, this website lists Research staff collaborations with outside companies if the Research staff member received any payment during the prior year or expects to receive any payment in the next year. The disclosures are based on information provided by the Research staff and other sources and are updated regularly. Current ownership interests and leadership positions are also listed. Further information may be available on individual company websites.
As of May 31, 2023, Dr. Dahia reported no relationships with healthcare industry.
By disclosing the collaborations of HSS Research staff with industry on this website, HSS and its Research staff make this information available to patients and the public, thus creating a transparent environment for those who are interested in this information. Further, the HSS Conflicts of Interest Policy does not permit payment of royalties on products developed by him/her that are used on patients at HSS.
