Early Diagnosis of Inflammatory Arthritis
An overview for primary care physicians and physical/occupational therapists, based on a presentation by Dr. Theodore R. Fields
If detected and treated in its early stages, the effects of inflammatory arthritis can be greatly diminished and the condition may even remit completely. The importance of proper diagnosis, particularly in the early stages of the disease, is often underestimated by primary care physicians, but it may prevent serious, lifelong arthritic complications for their patients.

This presentation addresses the following questions:
- What is the spectrum of IA?
- Why does it need to be diagnosed early?
- What treatments are available for early IA, especially RA?
- What is the role of the PCP and/or PT/OT in early IA?
Linked table of contents
How do you differentiate between mechanical arthritis and inflammatory arthritis?
Whereas mechanical arthritis (osteoarthritis) most commonly presents after the age of 50 and increases in frequency with age, inflammatory arthritis (IA) tends to involve a broader age spectrum, often striking patients in the peak working and child-rearing age group.
Mechanical arthritis is joint-localized, presents with spurs of new bone from the joints, and tends not be associated with systemic symptoms. However, patients with IA present with joint warmth (and occasional redness), soft swelling of the joints, and constitutional symptoms such as fever, weight loss, malaise, morning stiffness greater than 30 minutes and generalized fatigue. These symptoms are accompanied by extra-articular manifestations such as nodules, eye inflammation, and skin abnormalities.
A simple test of the metacarpal-phalangeal (MCP) joints can be performed to rule out osteoarthritis. In this test, the second through fourth metacarpo-phalangeal joints (see image, lower left) or the metatarso-phalangeal joints (see image, lower right) are squeezed together to test for tenderness. A positive result is an indication of the presence of inflammatory arthritis.
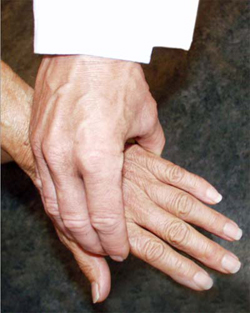

"Squeeze Test" of the MCP joints in the hand and MTP joints of the foot
What are the different types of inflammatory arthritis?
The most common type of IA is rheumatoid arthritis (RA) – 1% of the world's population is affected by this condition (three women to every man), and RA is one of the most common potentially treatable causes of disability in the Western world.
Other types of IA include:
- Psoriatic arthritis, in which scaly spots may appear on the skin, along with swelling in multiple joints and back stiffness, and at times, diffuse swelling of a finger or toe, giving the digit a "sausage-like" appearance.
- Gout and pseudogout, in which needle-like crystals of uric acid or rhomboid-shaped crystals of calcium pyrophosphate, respectively, form in cartilage and are released into the joint fluid, producing painful effects. Gout is most common in the first toe, where it meets the foot (the metatarso-phalangeal joint), but may also involve a number of other joints, such as the midfoot, ankle, knee and bursa of the elbow. Pseudogout occurs, generally, in an older population, and especially involves the ankles, knees, wrists and shoulders.
- Ankylosing spondylitis (AS) and related conditions affect the spine as well as peripheral joints. These conditions are associated with severe morning stiffness in the spine. In certain cases, other organs such as the eyes and heart may be affected.
- Parvovirus arthritis is a contagious, viral form of arthritis that can cause flu-like symptoms and rashes along with usual IA symptoms. Children with "fifth disease" due to parvovirus will often have a "slapped cheek" presentation of their rash, while their parents can present with a severe systemic inflammatory arthritis looking much like rheumatoid arthritis. Although parvovirus arthritis commonly resolves on its own within a few weeks, 10% of patients may experience ongoing symptoms.
- Lyme disease, caused by a spirochete (bacteria) contracted through the bite of an infected deer tick, produces IA symptoms in its later stages but can include early symptoms such as rashes, fatigue, chills, fever, headache, and a stiff, aching neck. Early antibiotic treatment is critical here. About 50% of patients are aware of a tick bite and/or the typical "target lesion" suggesting Lyme disease, but many cases need to be diagnosed based on a patient having been in an area known to be infected with the Lyme organism, presenting with symptoms consistent with Lyme disease. Blood testing is very useful once arthritis develops, but may be falsely negative very early in Lyme disease.
- Systemic lupus erythematosus (commonly called lupus or SLE) is an inflammatory condition which can involve essentially all systems of the body. Arthritis is one of the single most common ways for lupus to present, and lupus arthritis appears similar to the early stages of rheumatoid arthritis. Clues to lupus include a sun-sensitive skin rash, mouth sores, hair loss, chest pain with a deep breath, and a positive ANA blood test.
What are the warning signs of rheumatoid arthritis?
Multiple tests can be administered to detect the warning signs of RA. The proper diagnosis of RA can include the following:
- Symmetrical inflammatory arthritis (affecting both halves of the body in the same joint groups), especially in the small joints of the hands, associated with morning stiffness in the joints lasting over one half hour.
- Lab test results
- Positive rheumatoid factor
- Anti-CCP antibody elevation
- Sedimentation rate elevation
- X-ray changes
- Erosion of cartilage and/or bone in joints, and joint space narrowing – Radiographic erosions may develop early in the course of RA, and studies have shown that 25% of patients had erosions at their first visit. In addition, newer imaging techniques, such as MRI and ultrasound, are finding erosions significantly earlier in the course of RA than were found in prior studies using X-rays.
- Presence of rheumatoid nodules – These are very rare in the early stages of RA, but when present are helpful in diagnosis. It appears that with current improved therapies for RA, we are seeing much fewer nodules than were seen a decade ago.
Who gets rheumatoid arthritis?
RA is the most common form of inflammatory arthritis; as previously mentioned, it affects as high as 1% of the world's population, occurs three times more often in women than in men, and in most cases, develops in adults between the ages of 25 and 50. However, children and the elderly are also at risk.
What are the benefits of discovering inflammatory arthritis early?
IA requires early diagnosis; it can remit if left unchecked and undiagnosed in perhaps 5% of patients, but will much more likely evolve into a chronic inflammatory arthritis, most commonly RA. Conversely, early treatment can prevent future deformity and disability, and will likely help reduce collateral damage, such as atherosclerosis.
In early arthritis there is a "window of opportunity, during which the disease can be treated before irreversible joint erosions evolve. This window is usually open 3 to 6 months after the onset of the disease. For this reason, RA can be considered a "medical emergency."
In a meta-analysis of 14 trials by the American College of Rheumatology (ACR), patients suffering from a shorter duration of their disease were more likely to show improvement than long-term sufferers when properly diagnosed and treated. This finding supports the concept of a window of opportunity, in that early treatment improves not only short-term but long-term outcome.
What are the phases of rheumatoid arthritis?
The phases are early RA, transformation to chronic RA at about 12 weeks, and then chronic RA.
- Early RA – This term is generally applied to disease that is between a few weeks and one year in its progression. Some studies, however, consider "early arthritis" to be disease with six months of duration or less.
- The turning point to chronic RA begins after 12 weeks. This is when the aforementioned window of opportunity enters its most critical stage.
- Chronic RA sets in at more than one to two years, though some may consider any RA after six months to fall under this category.
What are the complications of untreated rheumatoid arthritis?
When RA is left untreated, joint destruction is highly likely, leading to functional disability and high risk of:
- inability to work
- psychosocial dysfunction
- comorbid conditions such as cardiovascular disease
- reduced life expectancy
- large economic loss, both to the individual and to society
What is the course of treatment for rheumatoid arthritis?
A multipronged approach is the watchword when it comes to RA treatment, including patient education, nonpharmacologic therapy, pain medications (such as NSAIDs), DMARDs and biologic agents, and/or surgery:
- Patient education: As early as possible after diagnosis, it is important for the patient to learn about his/her disease in order to anticipate its stages, symptoms, effects, treatments, and potential outcomes. The proper techniques for joint protection and other preventative measures are also important.
- Nonpharmacologic therapy can be extremely helpful in patients' efforts to overcome their disease – a physical therapist or occupational therapist is essential in aiding the patient's physical recuperation as concurrent pharmacologic treatment modalities are administered. The patient and therapist should focus on pain and spasm relief, strengthening and flexibility exercises, ambulation training, and assistive devices and splinting as indicated.
- Pain medications such as analgesics, NSAIDs (nonsteroidal anti-inflammatory drugs, such as ibuprofen or naproxen), and oral and intra-articular corticosteroids, used judiciously, can significantly improve the symptoms of patients with rheumatoid arthritis.
- DMARDs (disease-modifying antirheumatic drugs) and biologic agents: Read a review of the use of DMARDS (also called “slow acting agents”) and biologic agents for treating rheumatoid arthritis in Options for Treating Early Inflammatory Arthritis.
- Surgery: A variety of surgical procedures may be needed in patients with RA. If the arthritic process has been uncontrolled for a long period, sufficient joint damage may occur in a joint such as the hip or knee that a total hip replacement or knee replacement may be needed. Other procedures used at times in patients with RA include a "cleanout" or synovectomy of an inflamed joint, such as the knee, which can be done with an arthroscopic procedure. Repair of tendon ruptures by the inflammatory process, such as in the hand, can restore function.
What is the role of the primary care physician and physical therapist/occupational therapist in early inflammatory arthritis?
Primary care physicians and physical therapists should be attuned to the early warning signs of IA and recommend early consultation with a rheumatologist for work-up and possible early intervention. They can also educate the patient about the systemic and destructive nature of inflammatory joint disease and the "window of opportunity" presented by proper diagnosis of early arthritis.
Patients with early inflammatory arthritis need a "team," and this includes the primary care physician, the rheumatologist, and the physical and/or occupational therapist. While the patient gets started on medical therapy, it is crucial that their education process about living with the arthritis is ongoing. All such patients need to be taught an exercise and stretching program, and many need splinting as well as muscle-relaxing and motion-enhancing therapeutic modalities (for example, ultrasound, heat, etc.).
Conclusion
The spectrum of IA is broad, and RA is the most common type. It is a painful, disabling disease which shortens lifespan and can have a major impact on the systemic health and lifestyle of the patient. Early diagnosis and treatment of RA can markedly decrease disability and pain and very likely lengthen life, and the "window of opportunity" presented in the early stages of the disease must be recognized. Once the diagnosis of RA is made, disease modifying agents should be administered to essentially all RA patients.
RA is a condition that can be deforming, disabling and potentially life-shortening but, it is treatable, especially in its early stages. Treatment can still be quite effective even in the later stages of RA. Evidence has shown that patients with rheumatoid arthritis who are effectively treated can often avoid deformity and move their life span to the normal range. Stopping inflammation has been shown to not only stop joint swelling and pain, but also to decrease risk of heart complications. Primary care physicians and physical/occupational therapists can make a vital difference in their patients' lives if they know how to detect the early warning signs of arthritis and take the proper measures to make sure the patient has a health care team which can provide all the elements to stop the arthritis in its tracks.
Updated: 7/8/2022
Summary prepared by Mike Elvin
Authors
Attending Physician, Hospital for Special Surgery
Professor of Clinical Medicine, Weill Cornell Medical College
