Vasculitis
What is vasculitis?
Vasculitis refers to a group of conditions caused by inflammation in blood vessels. Any size blood vessel ranging from large arteries like the aorta to the smallest capillaries can be involved in vasculitis. When blood vessels are inflamed, the vessel itself can become damaged or blood flow through the vessel can be diminished resulting in damage to the target tissue or organ. Since blood vessels are responsible for supplying every part of the body, any area of the body can be affected by vasculitis. Vasculitis commonly affects the kidneys, lungs, nerves, skin and can even occur in blood vessels that supply the brain.
What are the different types of vasculitis?
There is a wide variety of vasculitis subtypes, including:
- Behcet's disease
- Buerger's disease (also known as thrombangiitis obliterans)
- cryoglobulinemia
- eosinophilic granulomatosis with polyangiitis (formerly known as Churg-Strauss syndrome)
- giant cell arteritis
- Granulomatosis with polyangiitis (formerly known as Wegener's granulomatosis)
- Henoch-Schönlein purpura
- Kawasaki disease
- microscopic polyangiitis
- polyarteritis nodosa
- Takayasu's arteritis
Buerger's disease (thrombangiitis obliterans) is a disorder where arteries and veins of the limbs (legs, arms, or both) become inflamed and clotted. This causes too little blood to flow to the limbs. The lack of blood circulation can lead to gangrene (tissue death), and the affected arm or leg may need to be amputated (surgically removed). Buerger’s disease is not common and is difficult to diagnose. Symptoms include severe pain in the lower arms or legs, particularly in hands and feet. Open sores may also appear in these parts of the body.
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's granulomatosis, is a form of vasculitis that most frequently affects the respiratory tract (sinuses, nose and lungs) and the kidneys. Other organs that can be affected are the skin, nerves, joints, eyes and ears. Its cause is unknown, and it can develop at any age but most commonly appears in people between the ages of 40 and 65.
What are the symptoms of vasculitis?
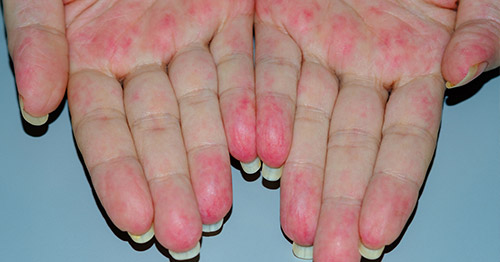
Symptoms of vasculitis are related to the part or parts of the body affected. For example, vasculitis affecting the vessels close to the skin’s surface are characterized by rash, whereas forms of the disease that affect blood vessels that supply the nerves may cause numbness or weakness in an affected extremity. Symptoms of vasculitis are related to size and location of blood vessels involved.
Commonly, patients with systemic vasculitis have constitutional symptoms including fever, night sweats and weight loss. Joint and muscle pain is also common in many vasculitic syndromes.
The severity of the condition ranges considerably from mild cases to those that are organ or life threatening.
What causes vasculitis?
Vasculitis can be caused by infections, medications, malignancies or be related to systemic autoimmune disease.
Vasculitis can occur as a disease unto itself, or in the context of an autoimmune disease such as rheumatoid arthritis, lupus or antiphospholipid syndrome. In those autoimmune diseases, the body perceives its own tissue as "foreign" and the immune system attacks the body’s own blood vessels.
Getting an accurate diagnosis by a rheumatologist is important because, although these diseases are treatable, early intervention may be needed to avoid significant, irreversible organ damage.
How is vasculitis diagnosed?
Vasculitis can be challenging to diagnosis as presentation can be very heterogenous and can mimic many other disorders. Blood and urine tests can be helpful in the diagnosis of certain forms of vasculitis. For other types of vasculitis, imaging like CT scan or MRI to visualize abnormal blood vessels can aid diagnosis. For many forms of vasculitis, a biopsy of the affected area can help confirm diagnosis as the actual inflamed blood vessel can be visualized under a microscope.
What is the treatment for vasculitis?
Treatment of vasculitis depends of the severity of disease and the organs involved. Most forms of vasculitis require immunosuppressive therapy. The goals of therapy are to tailor treatment to the individual, to put the disease into remission as soon as possible to prevent organ damage and to minimize treatment related side effects.
Some types of vasculitis can be treated with daily oral medicines, while other types require IV medication or weekly injections. Many forms of vasculitis require longer term therapy to maintain remission.
There are several research studies currently underway at HSS to better understand and treat many forms of systemic vasculitis. Physicians at HSS have participated in clinical trials that have resulted in approval of new therapies for multiple types of vasculitis, changing the treatment landscape and improving outcomes for people living with vasculitis. (Find a doctor at HSS who treats vasculitis.)
Can children get vasculitis?
Yes, vasculitis can affect people of all ages. In childhood, the most common form of vasculitis is Henoch-Schonlein purpura (also known as IgA vasculitis), this is typically a self-limited condition affecting skin, gastrointestinal tract, joints and occasionally the kidneys. Another form of vasculitis seen in children is called Kawasaki disease, where young children present with fevers, red eyes, characteristic rash and edema. Kawasaki may result in abnormalities of coronary arteries if not diagnosed and treated promptly.
What are the symptoms of vasculitis in children?
The symptoms a child has depend on the specific type of vasculitis and which blood vessels are affected. Many types of vasculitis have general symptoms such as prolonged fevers, fatigue, and weight loss. Children may have a characteristic rash that looks like small red dots or bruising (petechiae or purpura), or ulcers in their mouth or on their genitals. In children whose kidneys are affected by the disease, urine changes and high blood pressure may occur, and if the nervous system is affected, the child may have seizures, stroke, or other neurological changes. Other concerning symptoms include trouble breathing, coughing up blood, or bloody diarrhea. If you are ever concerned for your child’s immediate safety, bring him or her to the emergency room for an evaluation. Depending on the manifestations of the disease, in addition to standard immunotherapy treatments, children may require other types of medication, including antihypertensives for blood pressure control.
Learn more about vasculitis in the articles below.
Vasculitis articles for patients
Vasculitis articles for healthcare professionals
Articles related to vasculitis
Vasculitis Clinical Trials
Vasculitis Patient Stories
In the news
References
- Lally L, Sammaritano LR. Vasculitis in antiphospholipid syndrome. Rheum Dis Clin North Am. 2015;41(1):109-23, ix. doi: 10.1016/j.rdc.2014.09.009. PMID: 25399943.
- Lally L, Spiera R. B-cell-targeted therapy in systemic vasculitis. Curr Opin Rheumatol. 2016 Jan;28(1):15-20. doi: 10.1097/BOR.0000000000000235. PMID: 26599379.
- Lally L, Spiera RF. Biomarkers in ANCA-associated vasculitis. Curr Rheumatol Rep. 2013 Oct;15(10):363. doi: 10.1007/s11926-013-0363-x. PMID: 23955064.
- Lally L, Spiera R. Current therapies for ANCA-associated vasculitis. Annu Rev Med. 2015;66:227-40. doi: 10.1146/annurev-med-011514-023051. Epub 2014 Oct 17. PMID: 25341007.
- Lally L, Spiera RF. Pulmonary vasculitis. Rheum Dis Clin North Am. 2015 May;41(2):315-31. doi: 10.1016/j.rdc.2015.01.004. Epub 2015 Feb 27. PMID: 25836645.
Updated: 6/6/2023
Reviewed and updated by Lindsay S. Lally, MD and Sarah Faith Taber, MD